REVISION NOTES
IGCSE Edexcel Chemistry
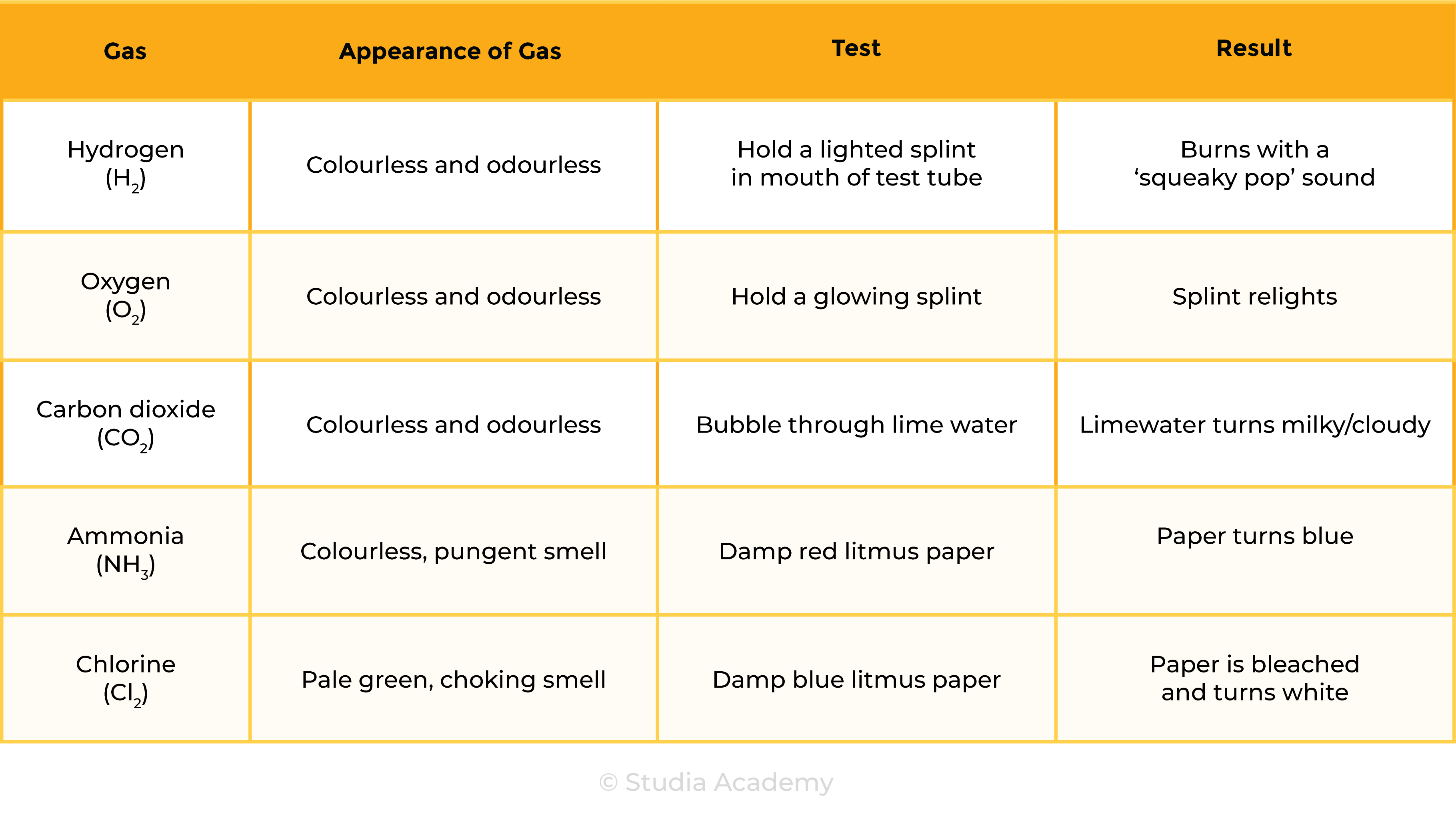
2.8 Chemical Tests
2.8.1 Describe tests for these gases:
- Hydrogen
- Oxygen
- Carbon dioxide
- Ammonia
Test of Gases
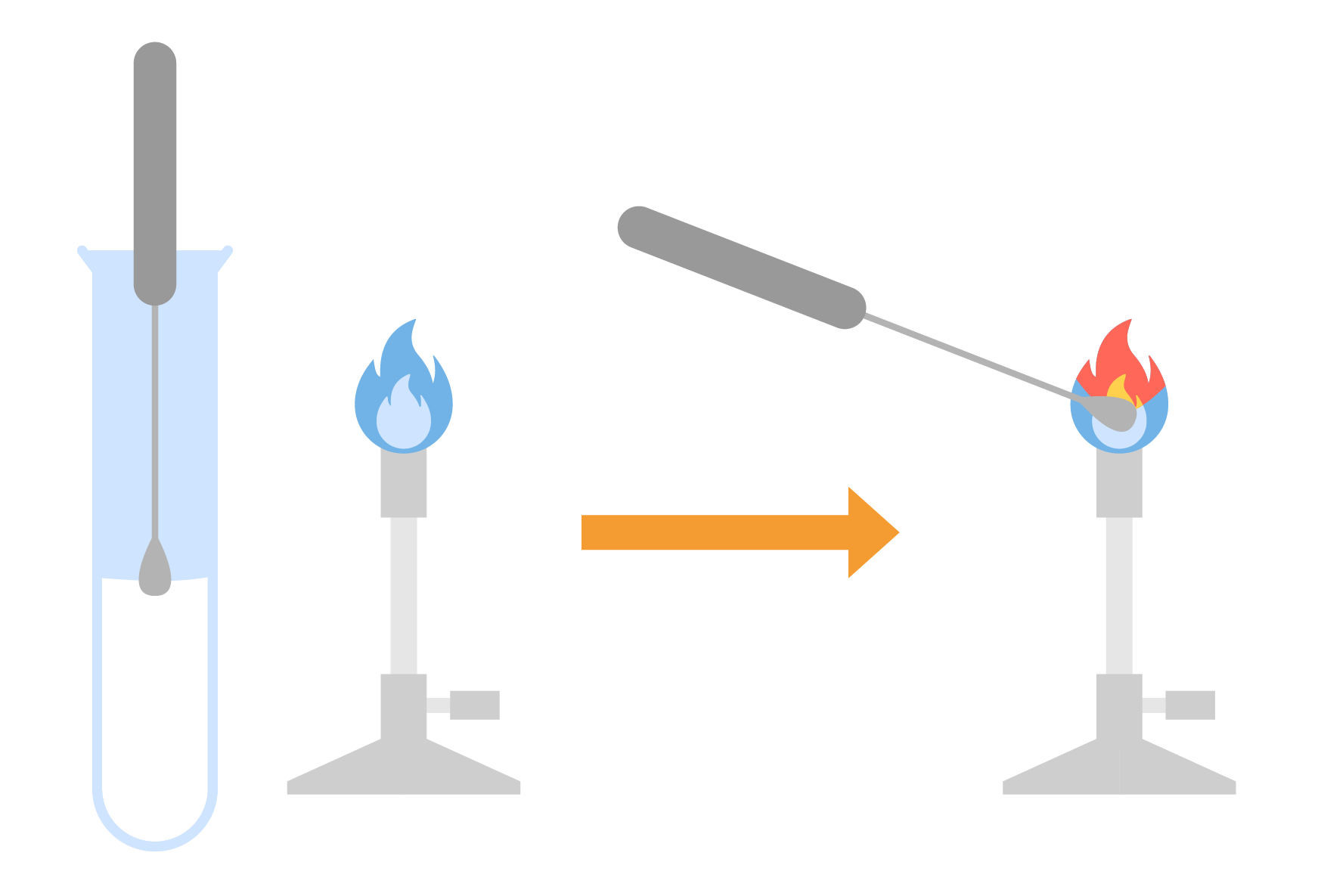
2.8.2 Describe how to carry out a flame test
FLAME TEST
- When metal ions are heated in a flam, they produce a distinctive colour
- The flame test is thus used to identify metal ions by observing the colour of the flame produced
Methods
- Dip a metal wire loop made from unreactive metal (nichome or platinum) in concentrated acid
- Then hold it in the blue flame of a Bunsen burner until there is no colour change
- This cleans the wire loop to avoid contamination
- Dip the metal wire loop into the solid sample
- It is then placed in the edge of the blue Bunsen burner flame
- It should not be heated for too long as it would glow red and affect the observation of flame colour
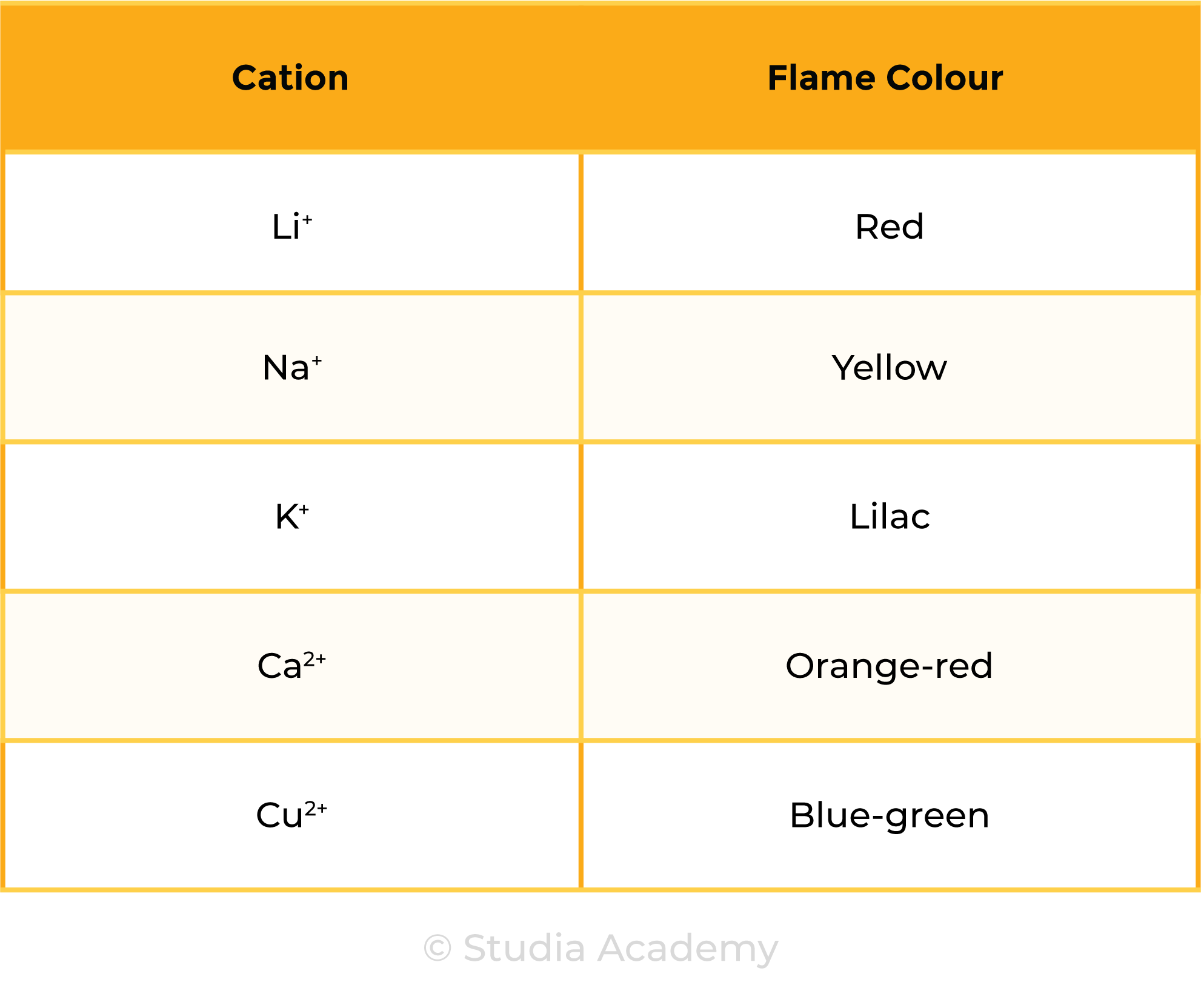
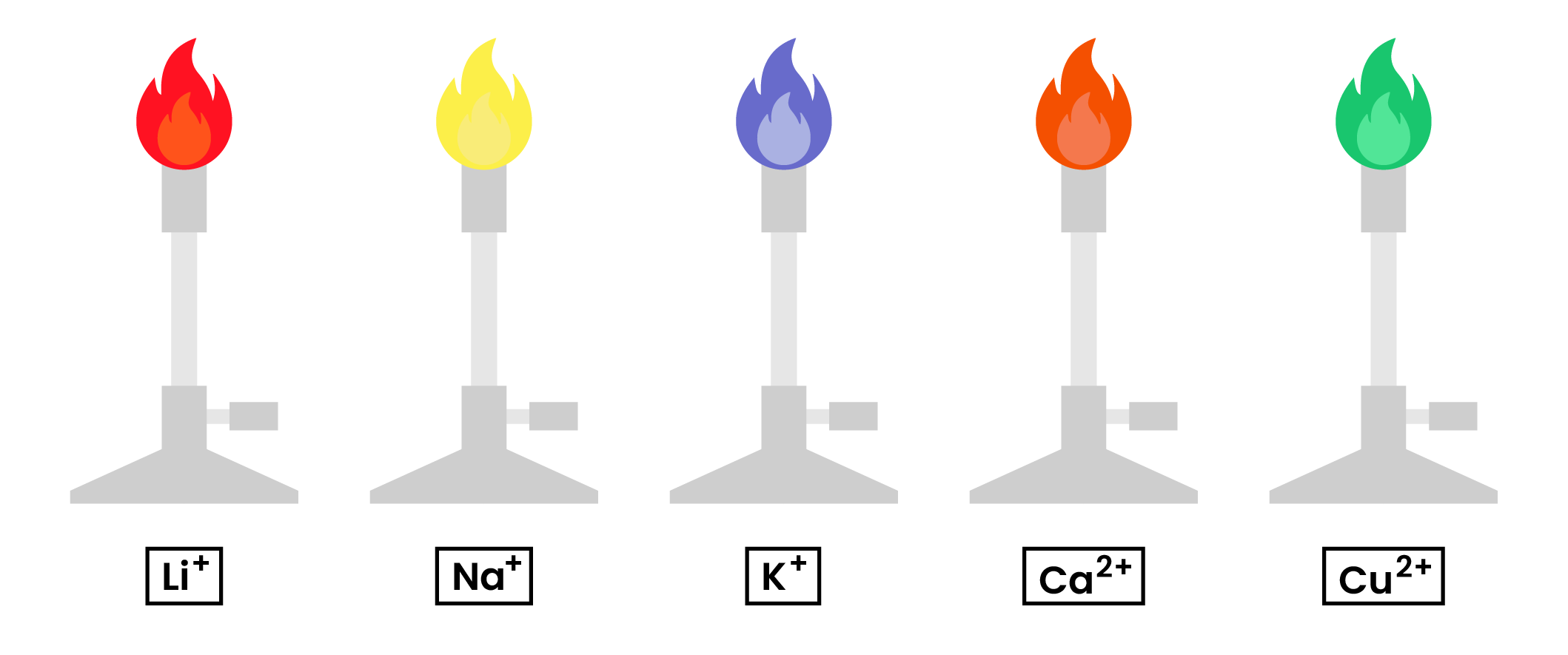
2.8.3 Know the colours formed in flame tests for these cations:
- Li+ is red
- Na+ is yellow
- K+ is lilac
- Ca2+ is orange-red
- Cu2+ is blue-green.
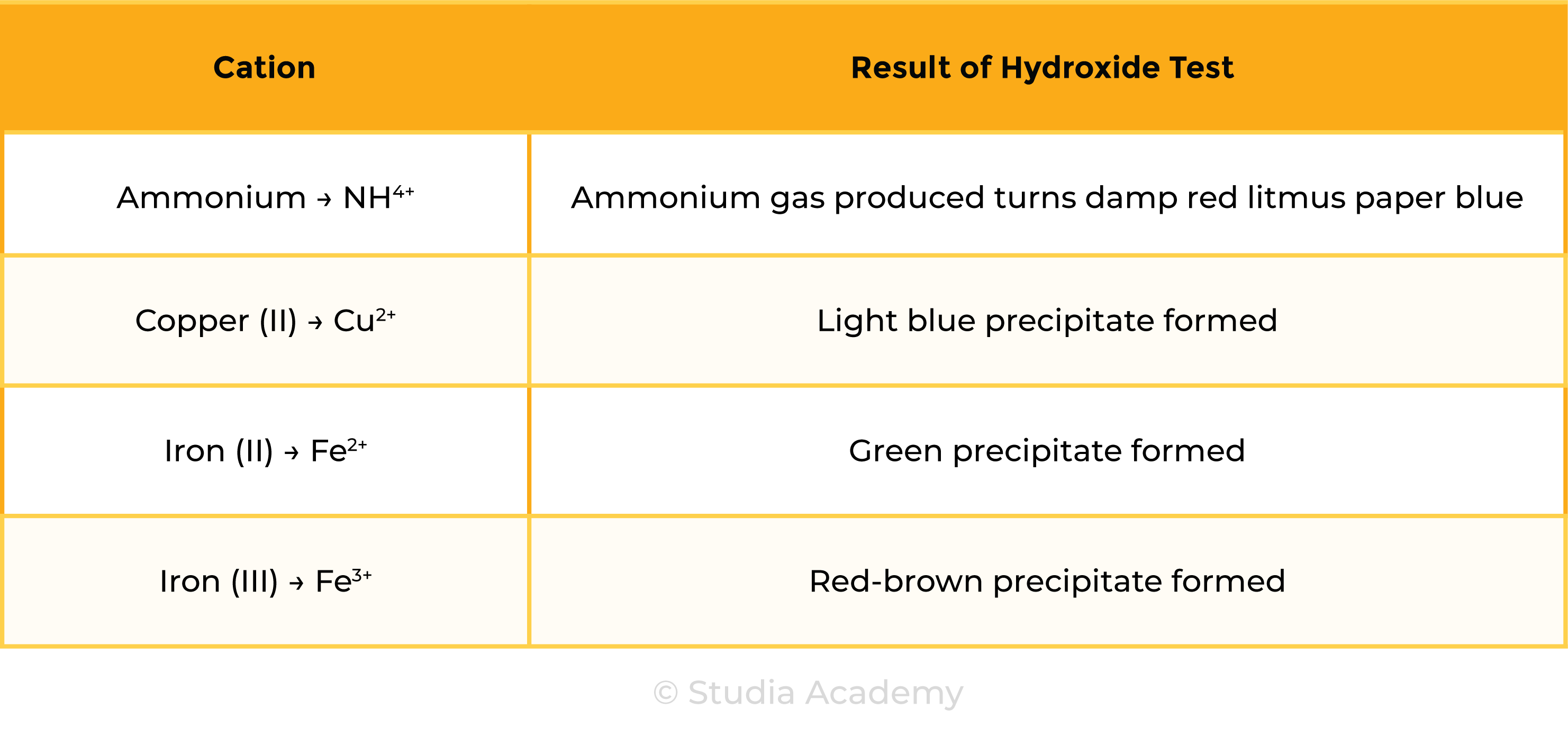
2.8.4 Describe tests for these cations:
- NH4+ using sodium hydroxide solution and identifying the gas evolved
- Cu2+, Fe2+ and Fe3+ using sodium hydroxide solution.
Test for Cations
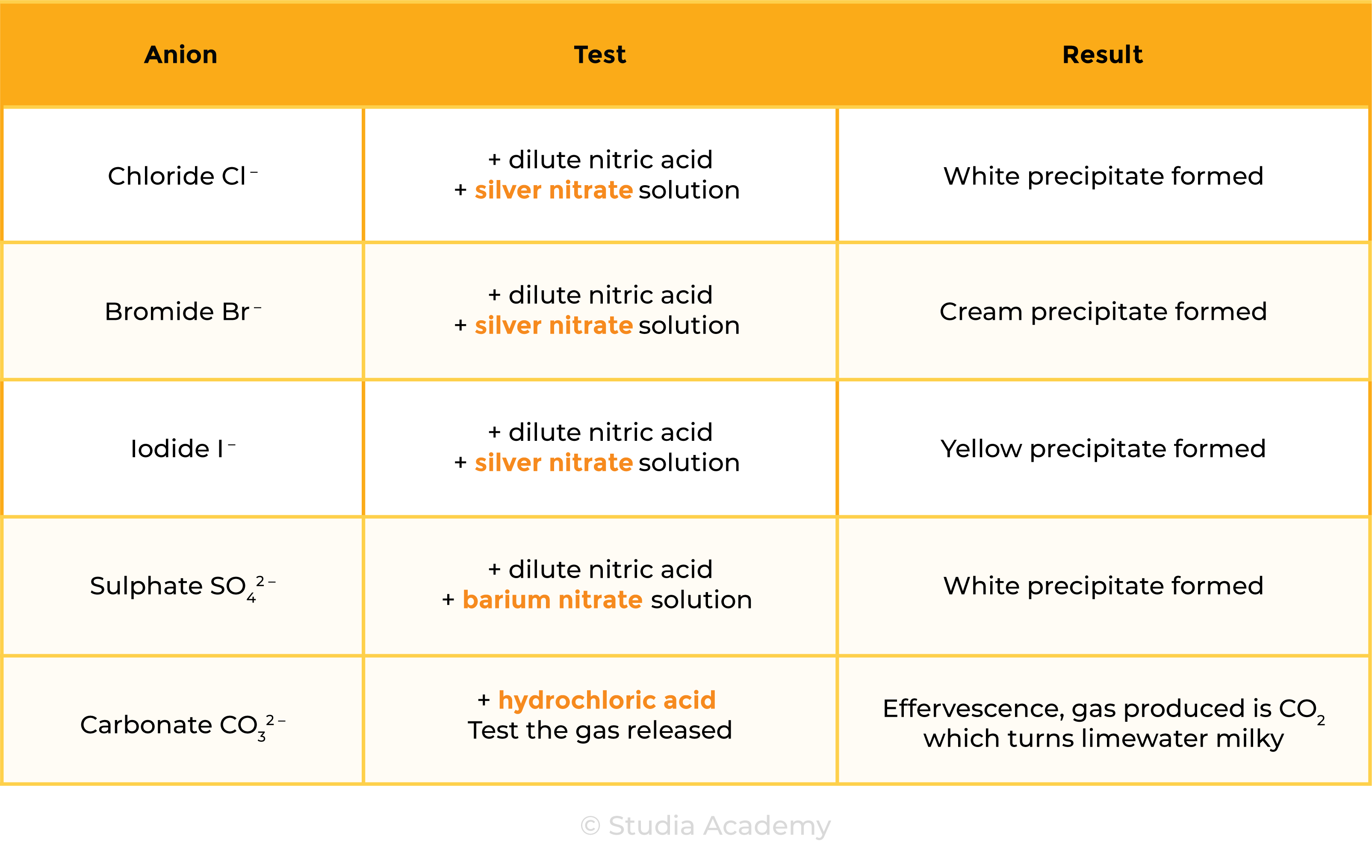
2.8.5 Describe tests for these anions:
- Cl–, Br– and I– using acidified silver nitrate solution
- SO42– using acidified barium chloride solution
- CO32– using hydrochloric acid and identifying the gas evolved.
Test for Anions
Nitric acid is added first to react with and remove any other ions (e.g. carbonate ions) that may be present that would give a confusing precipitate with silver nitrate. (E.g. If carbonate ions are present, a white precipitate of silver carbonate would be produced which gives a false result of chloride ions)
2.8.6 Describe a test for the presence of water using anhydrous copper(II) sulfate
TESTS FOR WATER (CHEMICAL)
- Anhydrous copper(II) sulphate turns from white to blue on the addition of water
2.8.7 Describe a physical test to show whether a sample of water is pure
TETS FOR WATER (PHYSISCAL)
- Check boiling point of water


