REVISION NOTES
IGCSE Edexcel Chemistry
2.6 Acids, Alkalis and Titrations
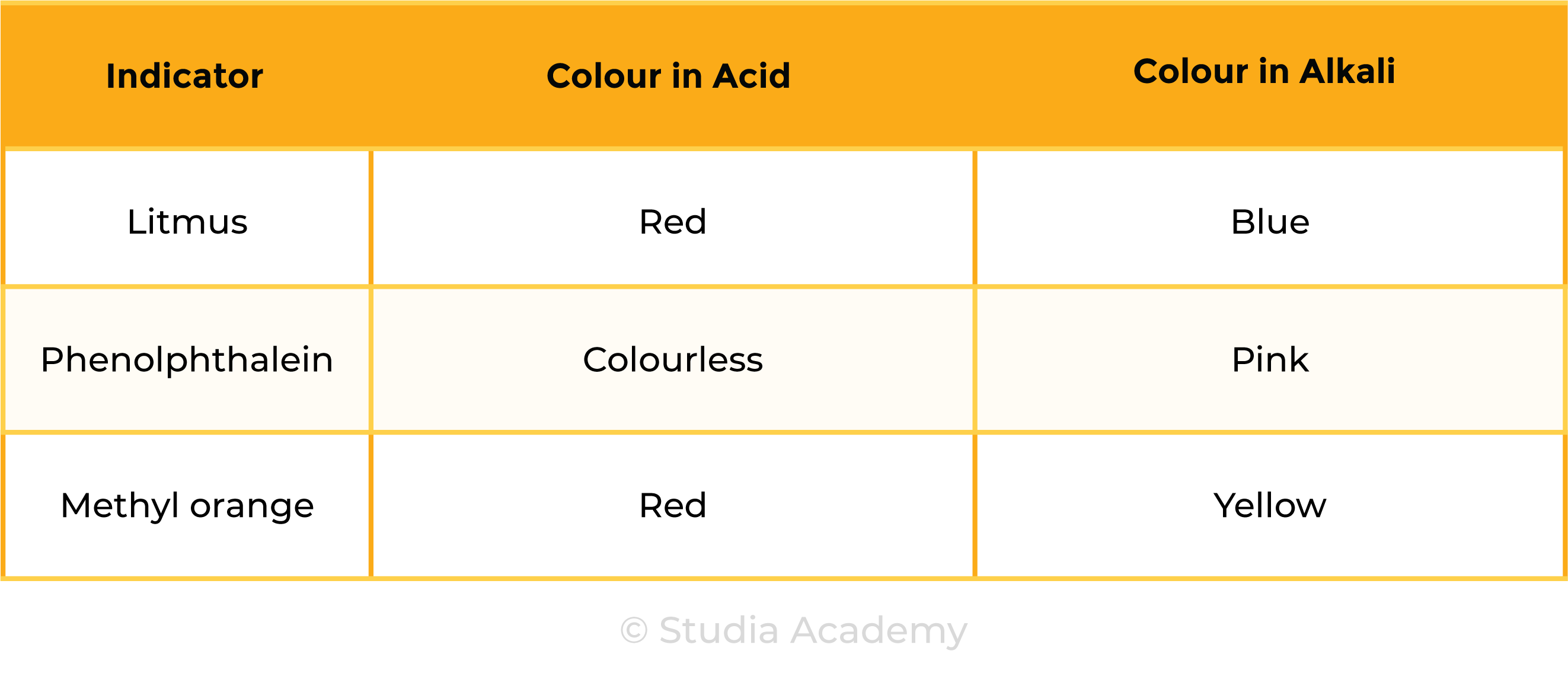
2.6.1 Describe the use of litmus, phenolphthalein and methyl orange to distinguish between acidic and alkaline solutions
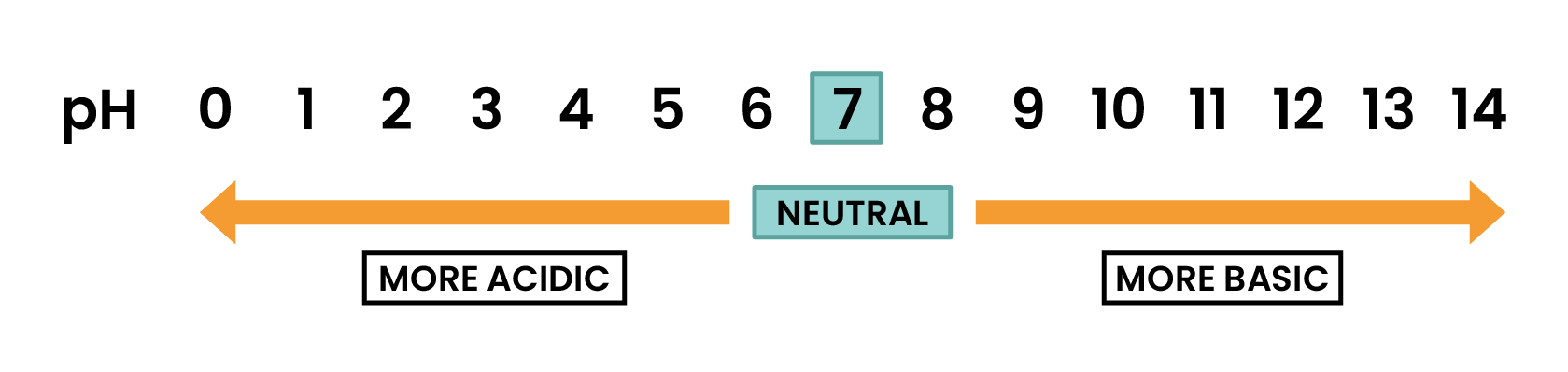
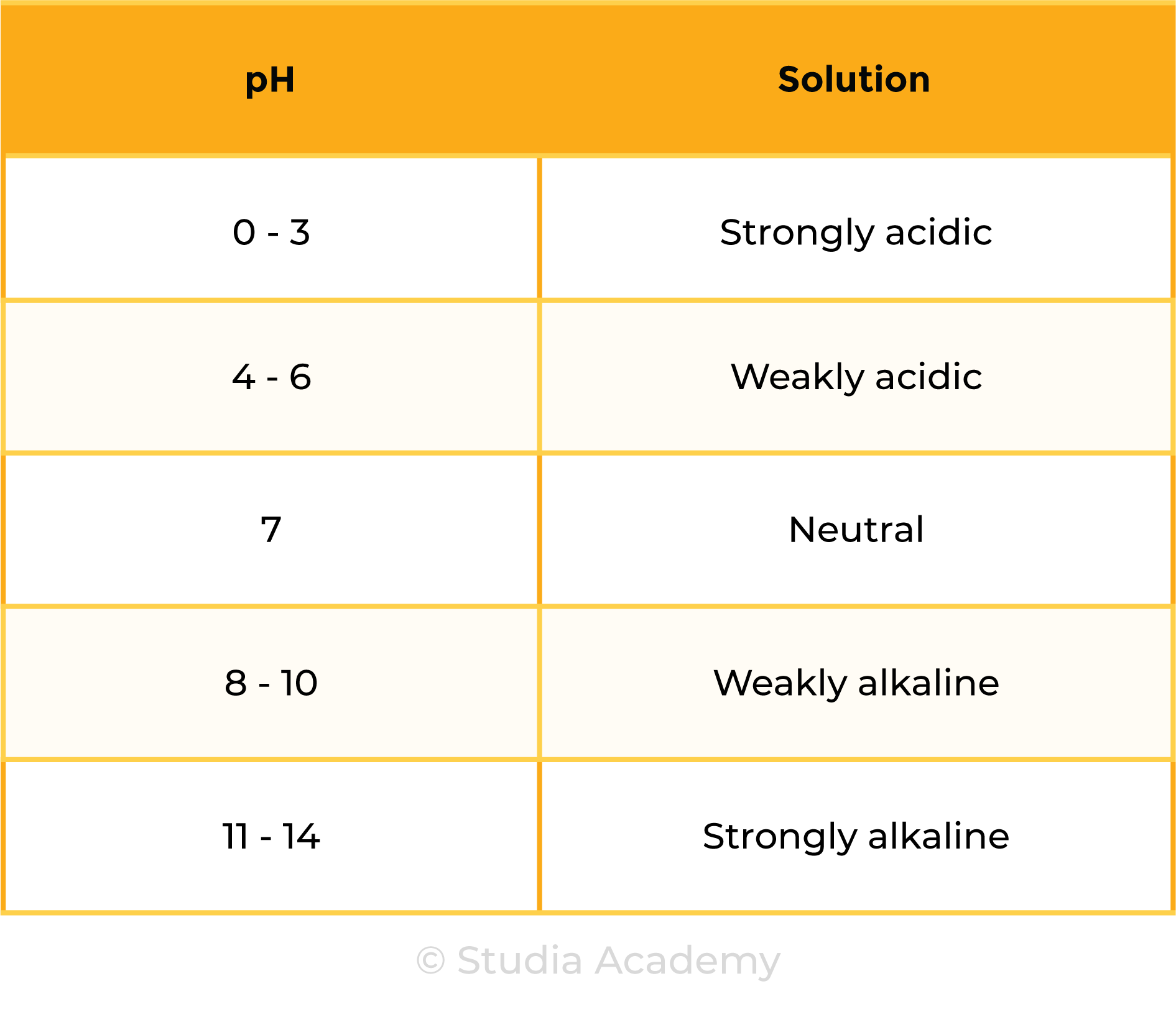
2.6.2 Understand how to use the pH scale, from 0–14, can be used to classify solutions as strongly acidic (0–3), weakly acidic (4–6), neutral (7), weakly alkaline (8–10) and strongly alkaline (11–14)
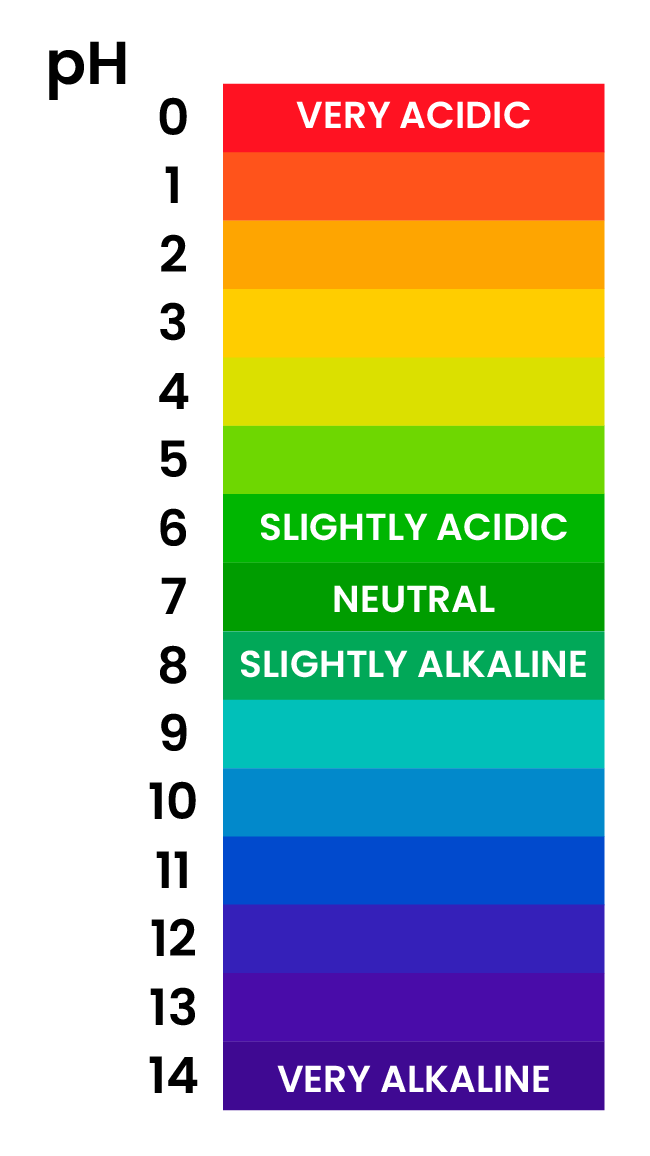
2.6.3 Describe the use of universal indicator to measure the approximate pH value of an aqueous solution
UNIVERSAL INDICATOR
- Universal indicator is a wide range indicator
- Can give only an approximate value for pH
- A few drops are added to the solution and the colour is matched with a colour chart which indicates the pH which matches with specific colours
2.6.4 Know that acids in aqueous solution are a source of hydrogen ions and alkalis in a aqueous solution are a source of hydroxide ions
ACIDS
- Source of hydrogen ions H+
- The presence of H+ ions is what makes a solution acidic
ALKALIS
- Source of hydroxide ions OH–
- The presence of OH– ions is what makes the solution an alkali
2.6.5 Know that alkalis can neutralise acids
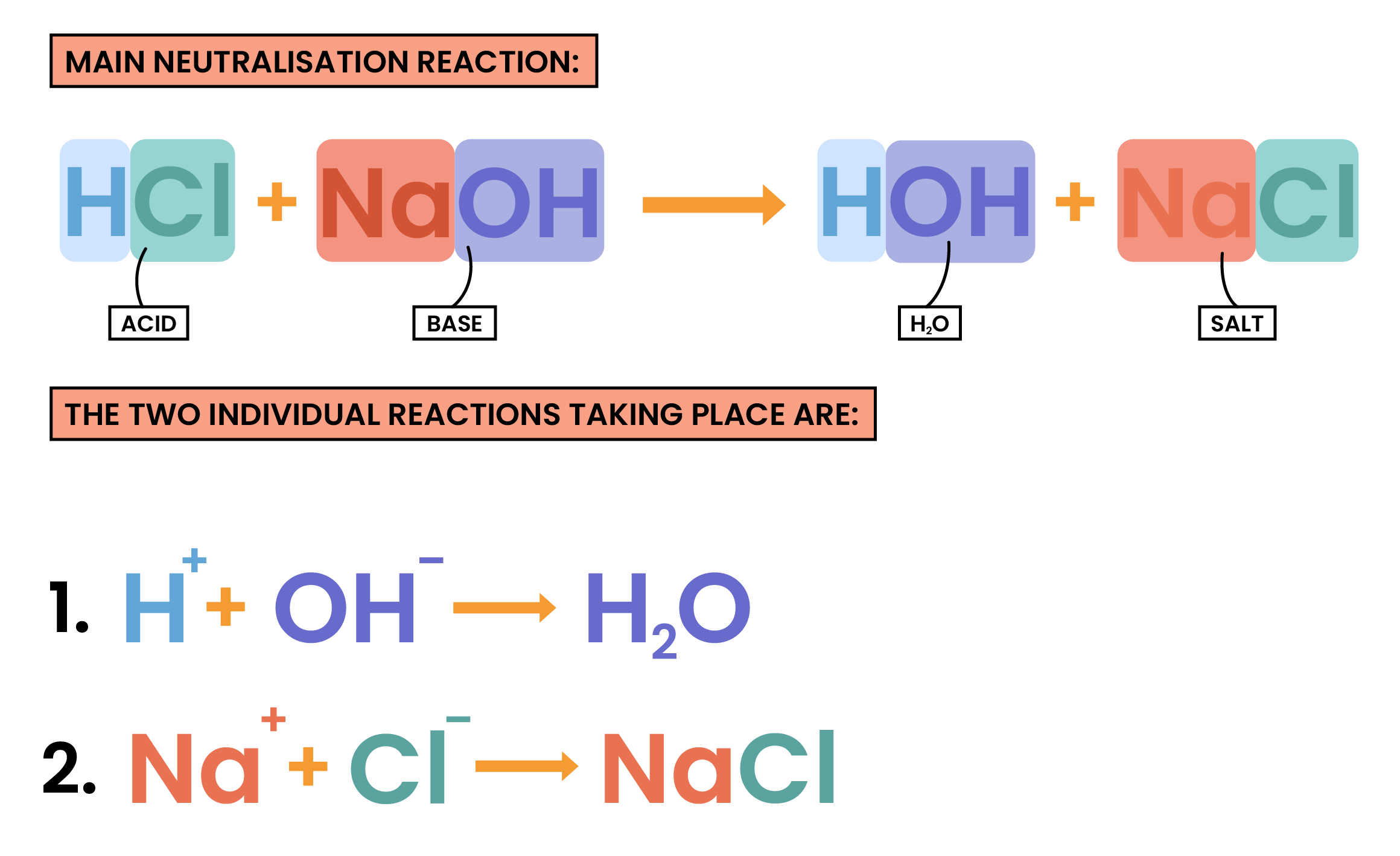
NEUTRALISATION
- A neutralisation reaction occurs when an acid reacts with an alkali
- H+ ions from acids and OH– ions from alkalis react to produce water
2.6.6C Describe how to carry out an acid-alkali titration
ACID-ALKALI TITRATION
- Titrations are a method of analysing the concentration of solutions
- They can determine exactly how much alkali is needed to neutralise a quantity of acid – and vice versa
- It relies on the colour change of an acid–alkali indicator
- Calculations will be carried out for mole, volume and concentrations
- Titrations can also be used to prepare salts
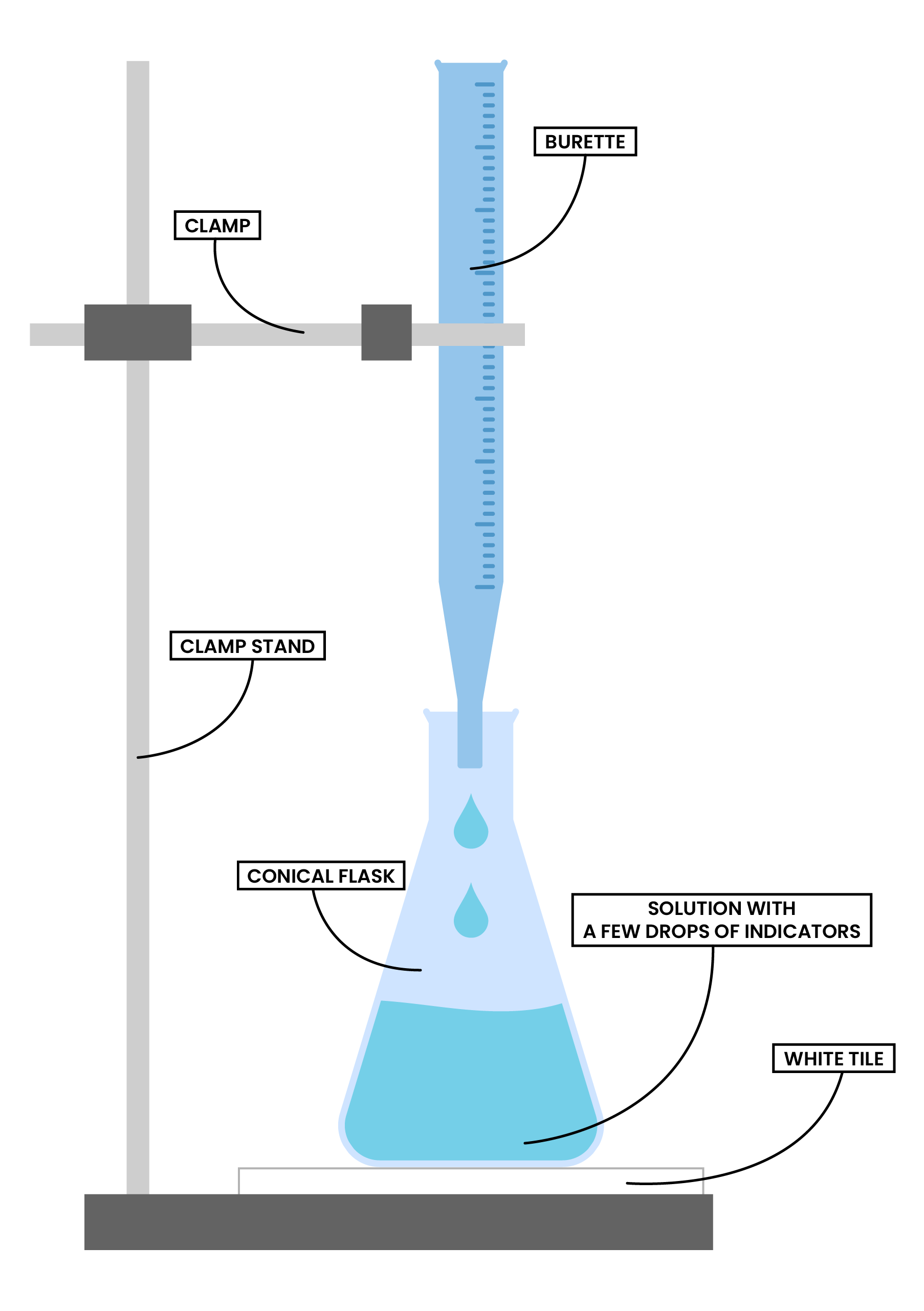
HOW TO CARRY OUT TITRATION
- Use a measuring cylinder to measure a known volume of an acid, add it to a conical flask
- Add a few drops of indicator to the same conical flask
- Place the conical flask on a white tile (for easier observation of colour change)
- Fill the burette with a known concentration of an alkali
- Record the initial volume of the alkali
- Slowly add the alkali to the flask in small portions, swirl the flask in between to ensure reaction fully takes place
- Immediately close the tap when the colour changes (it means it reaches the end-point)
- Record the final volume of the alkali in the burette

