REVISION NOTES
IGCSE Edexcel Chemistry
2.4 Reactivity Series
2.4.1 Understand how metals can be arranged in a reactivity series based on their reactions with:
- Water
- Dilute hydrochloric or sulfuric acid
REACTIVITY SERIES OF METAL
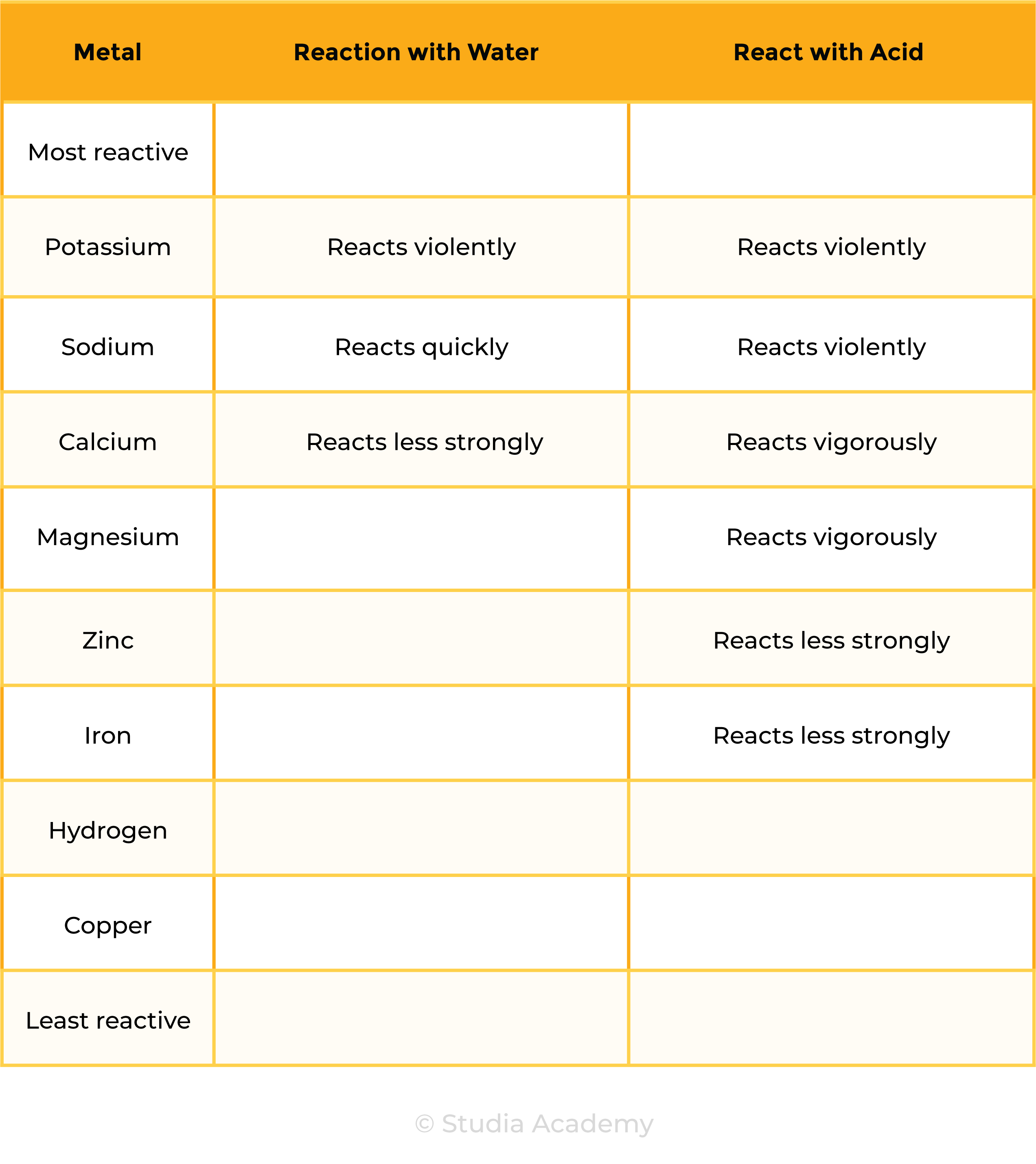
- The reactivity series of metal can be determined by analysing their reactions with water and acids
- Metals react with water and acids
- Based on the observation made of the reactions, metals are placed in order of reactivity
Reaction 1: Metal + Water
Metal + Water → Metal hydroxide + Hydrogen
Ca (s) + 2H2O (l) → Ca(OH)2 (aq) + H2 (g)
Calcium + Water → Calcium hydroxide + Hydrogen
Reaction 2: Metal + Acid
Metal + Acid → Salt + Hydrogen
2K (s) + 2HCl (aq) → 2KCl (s) + H2 (g)
Potassium + Hydrochloric acid → Potassium chloride + Hydrogen
- Only metals above hydrogen in the reactivity series will react with acids
- The more reactive the metal, the more vigorous the reaction
2.4.2 Understand how metals can be arranged in a reactivity series based on their displacement reactions between:
- Metals and metal oxides
- Metals and aqueous solutions of metal salts
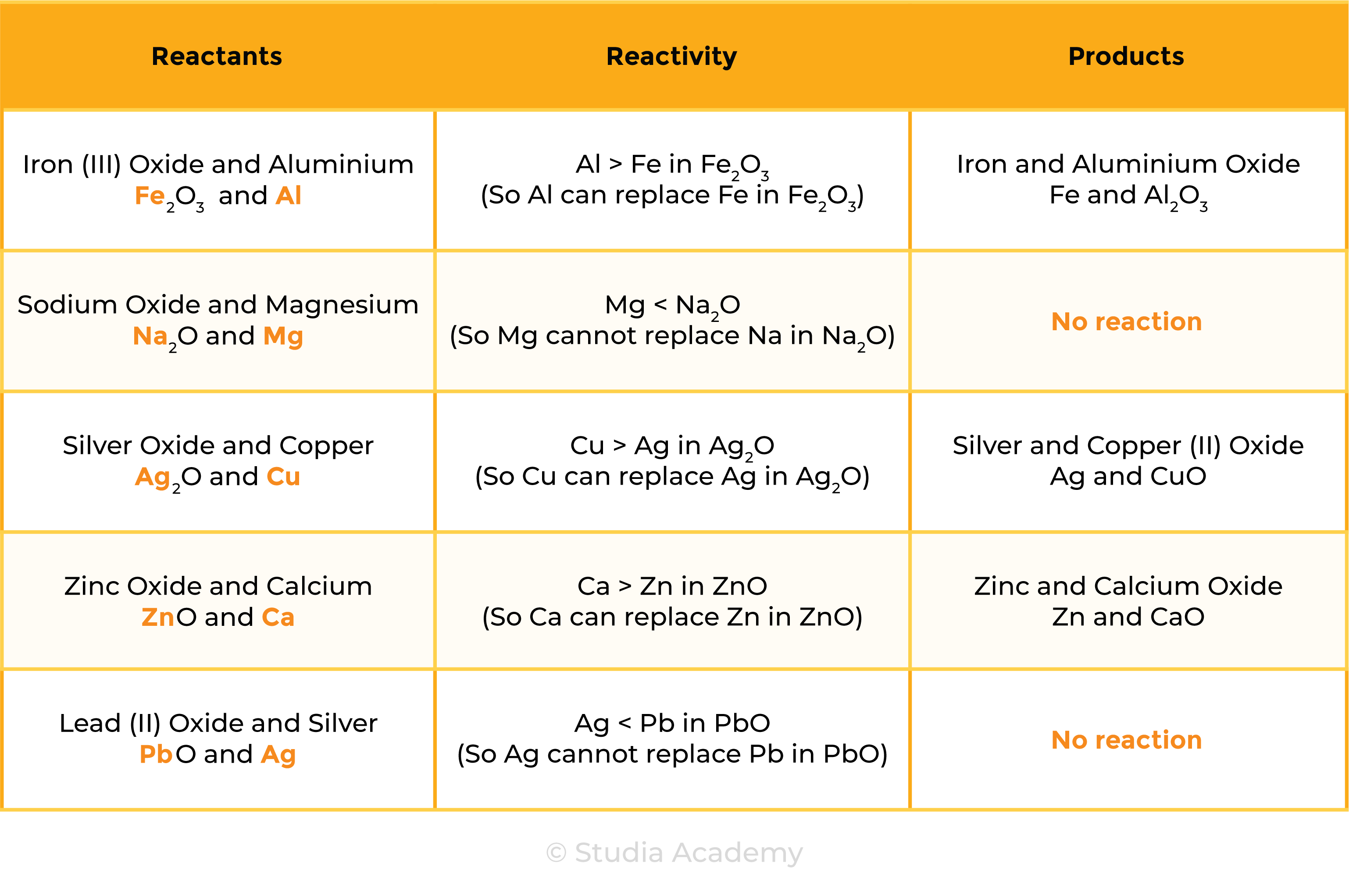
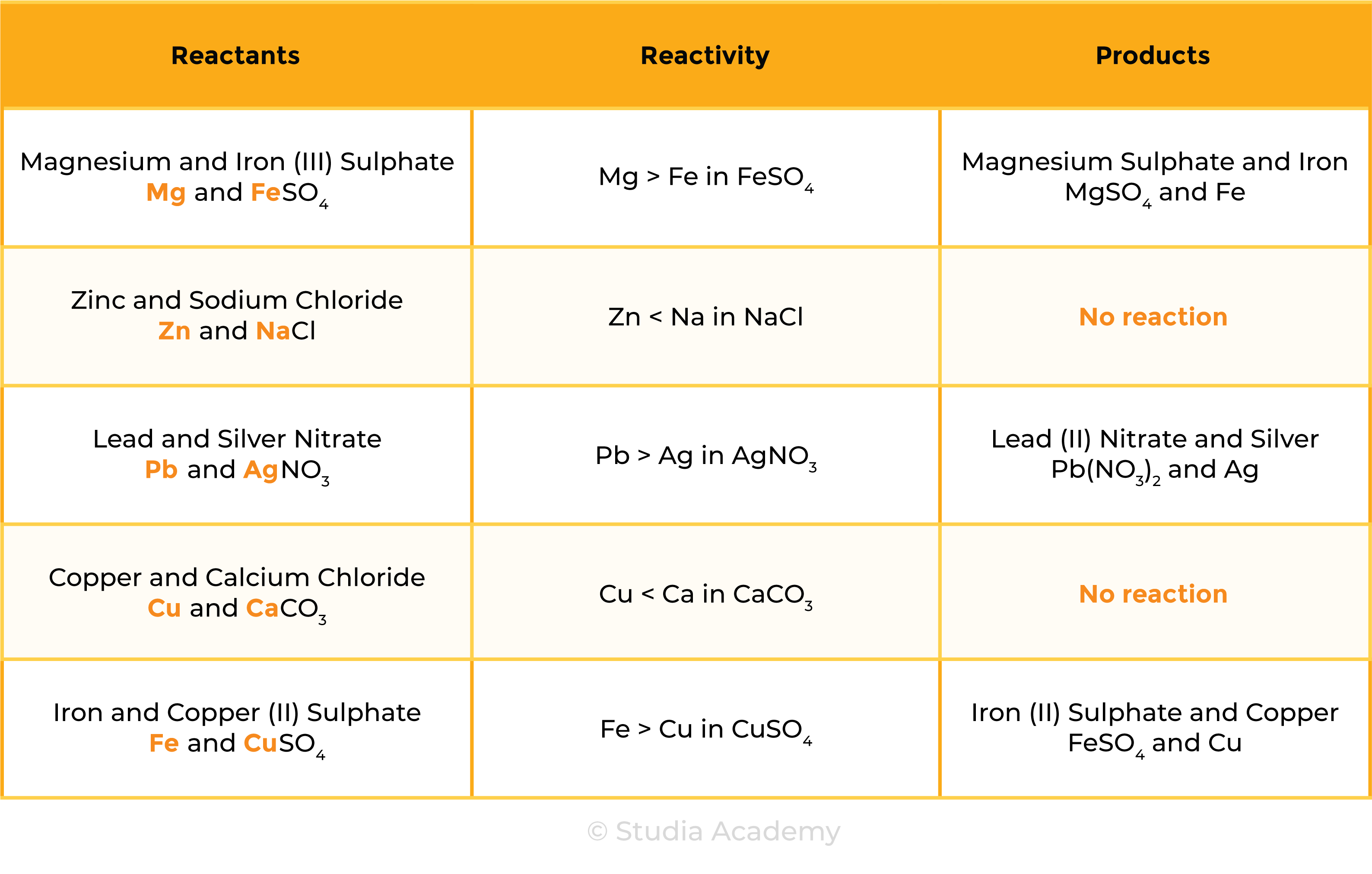
The reactivity of metal decreases going down the reactivity series
- A more reactive metal will displace a less reactive metals from its compounds
- Two reactions will be discussed:
- Metal and metal oxide
- Metal and aqueous solutions of metal salts
REACTION 1 METAL + METAL OXIDE
- Zn + CuO → ZnO + Cu
- According to the reactivity series, Zn is more reactive than Cu
- Therefore, Zinc can displace Cu in CuO and form ZnO
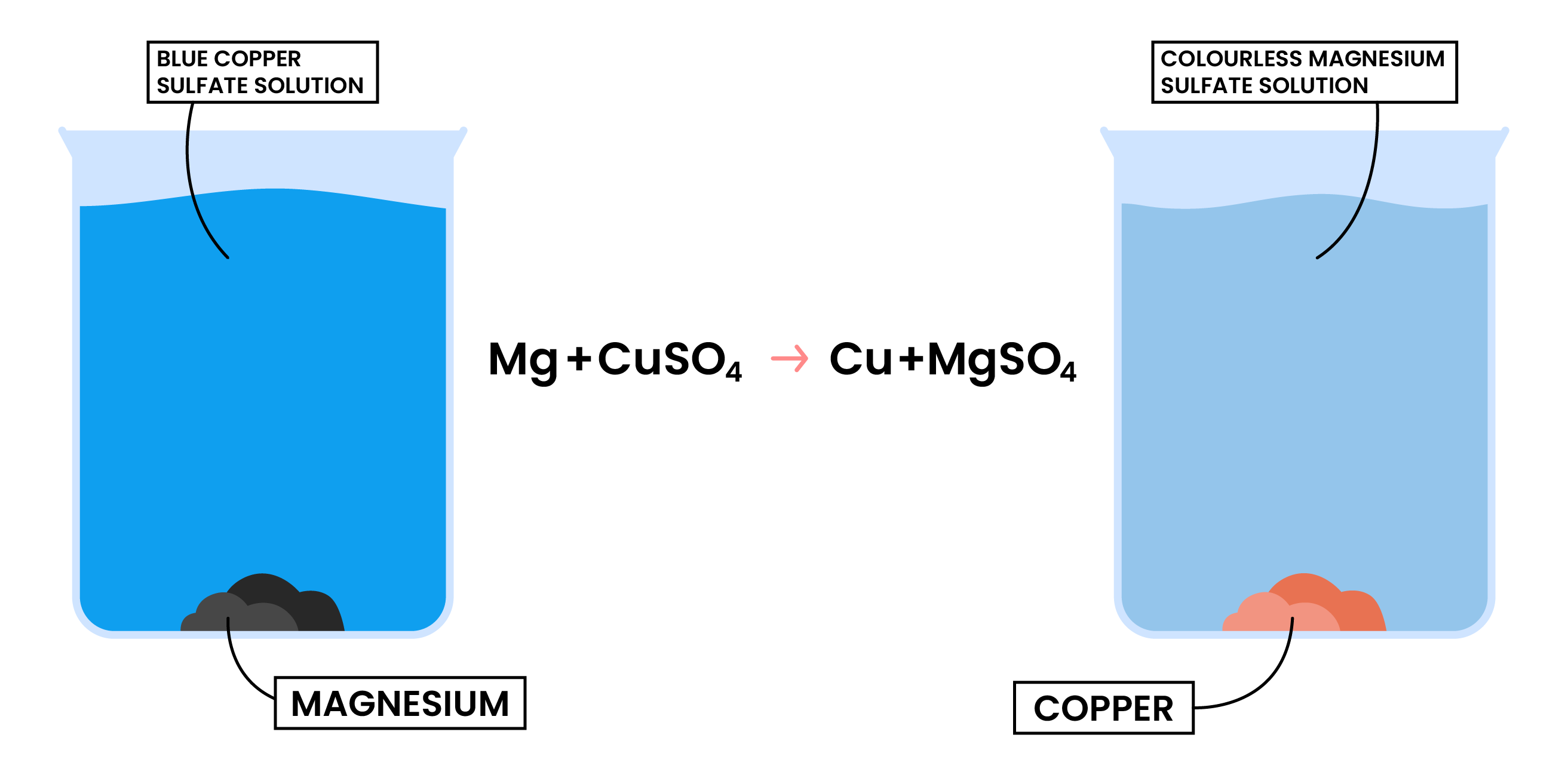
REACTION 2 METAL + AQUEOUS SOLUTION OF METAL SALTS
- A more reactive metal displaces the less reactive metal in its aqueous solution
- The more reactive metal slowly disappears from the mixture
- Mg + CuSO4 → MgSO4 + Cu
- Mg is more reactive than Cu, so Mg can displace Cu in CuSO4
- The blue colour of CuSO4 fades as colourless MgSO4 solution is formed
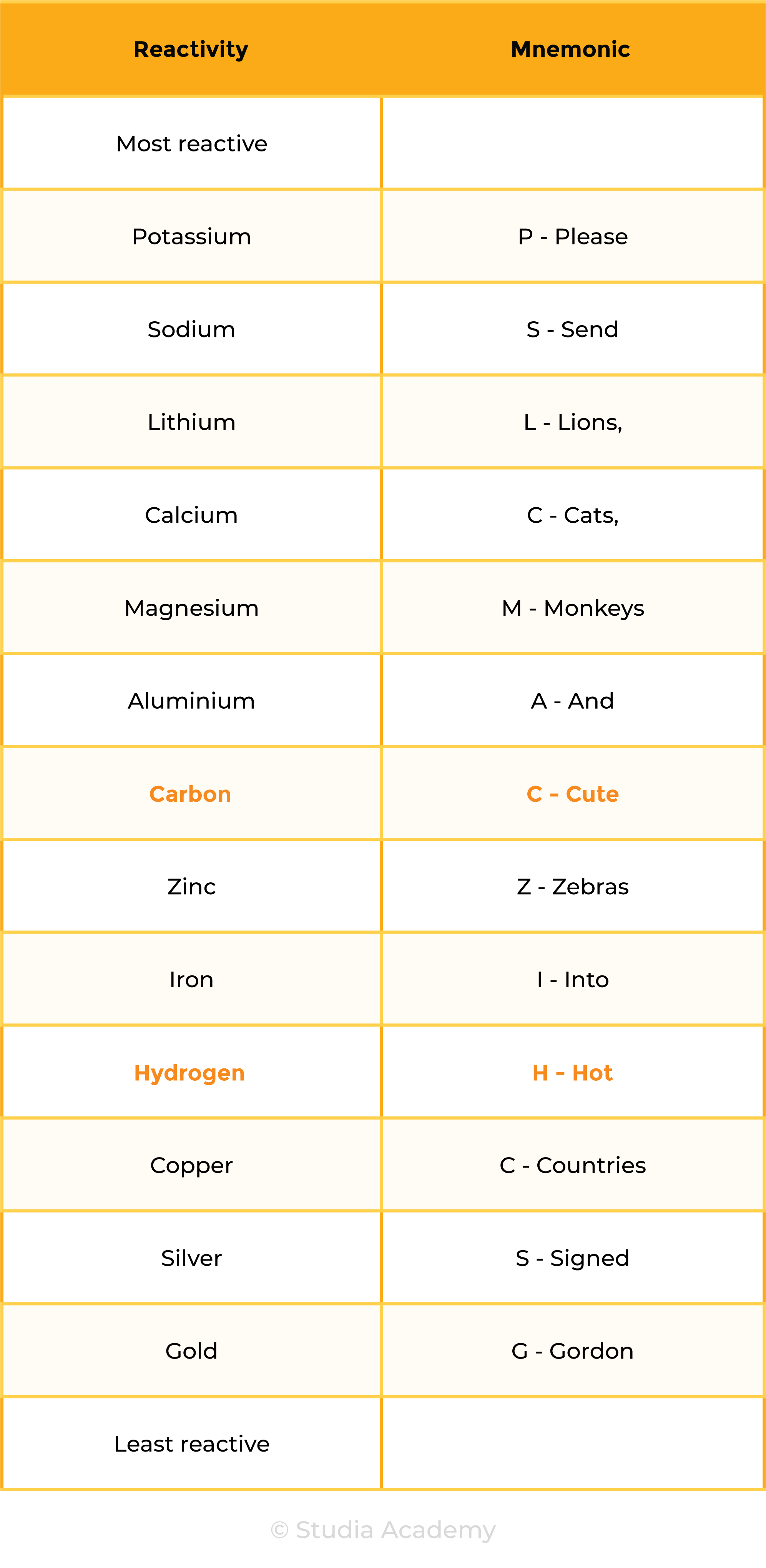
2.4.3 Know the order of reactivity of these metals: potassium, sodium, lithium, calcium, magnesium, aluminium, zinc, iron, copper, silver, gold
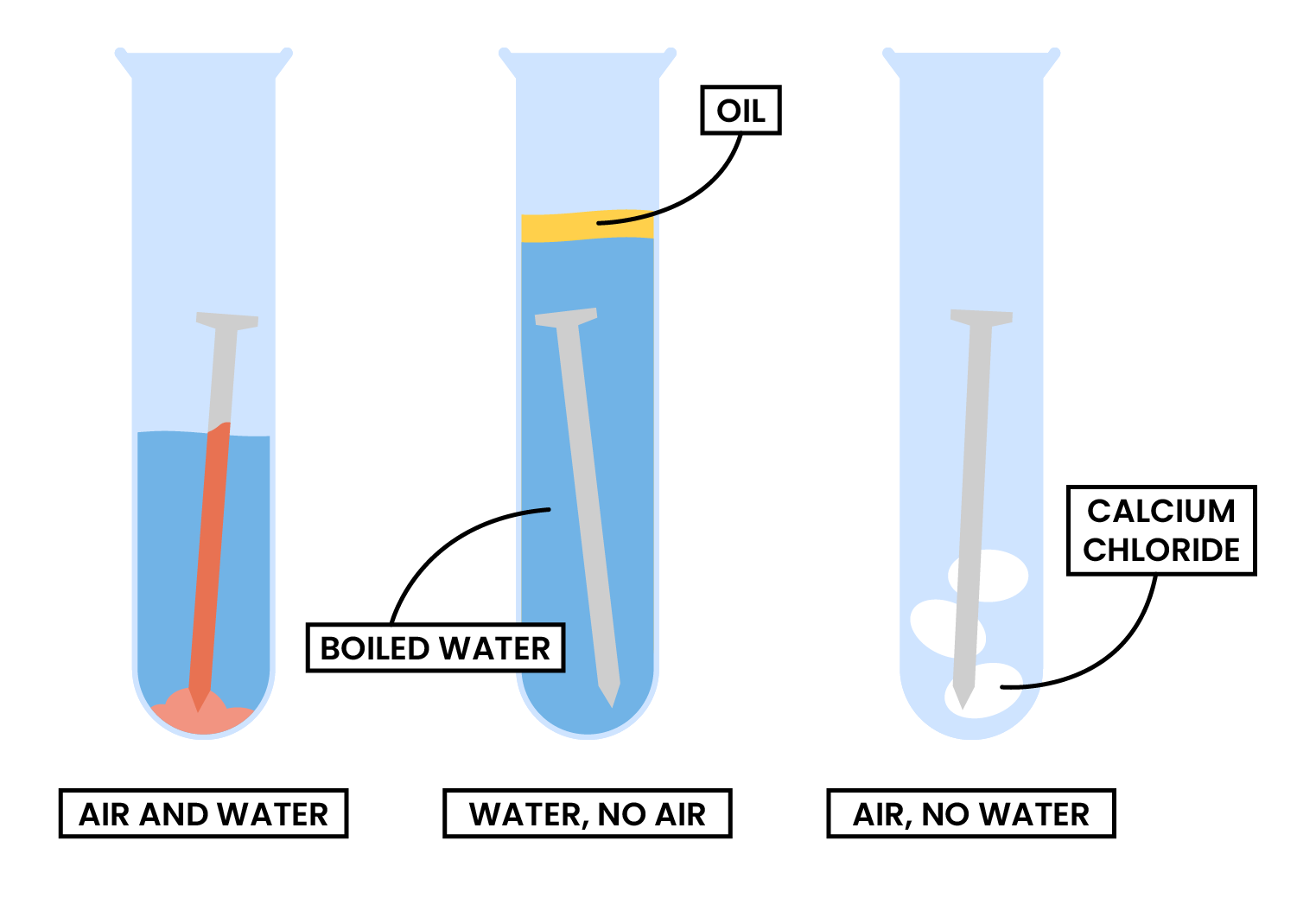
2.4.4 Know the conditions under which iron rusts
RUSTING OF IRON
- Rusting is a chemical reaction between iron, oxygen and water
- Hydrated iron (III) oxide is formed
- Required conditions:
- Oxygen
- Water
- Rusting occurs faster in salty water as sodium chloride speeds up the reaction
Iron + Water + Oxygen → Hydrated Iron(III) Oxide
4Fe (s) + 3O2 (g) + xH2O (l) → 2Fe2O3・xH2O (s)
2.4.5 Understand how the rusting of iron may be prevented by:
- Barrier methods
- Galvanising
- Sacrificial protection
METHOD 1 BARRIER METHOD
- Rust can be prevented by coating iron with barriers
- To prevent iron from contacting with water and oxygen
- Common barrier methods:
- Grease
- Oil
- Paint
- Plastic
- However, if the coatings are washed away or scratched, the iron is again exposed to water and oxygen and will rust
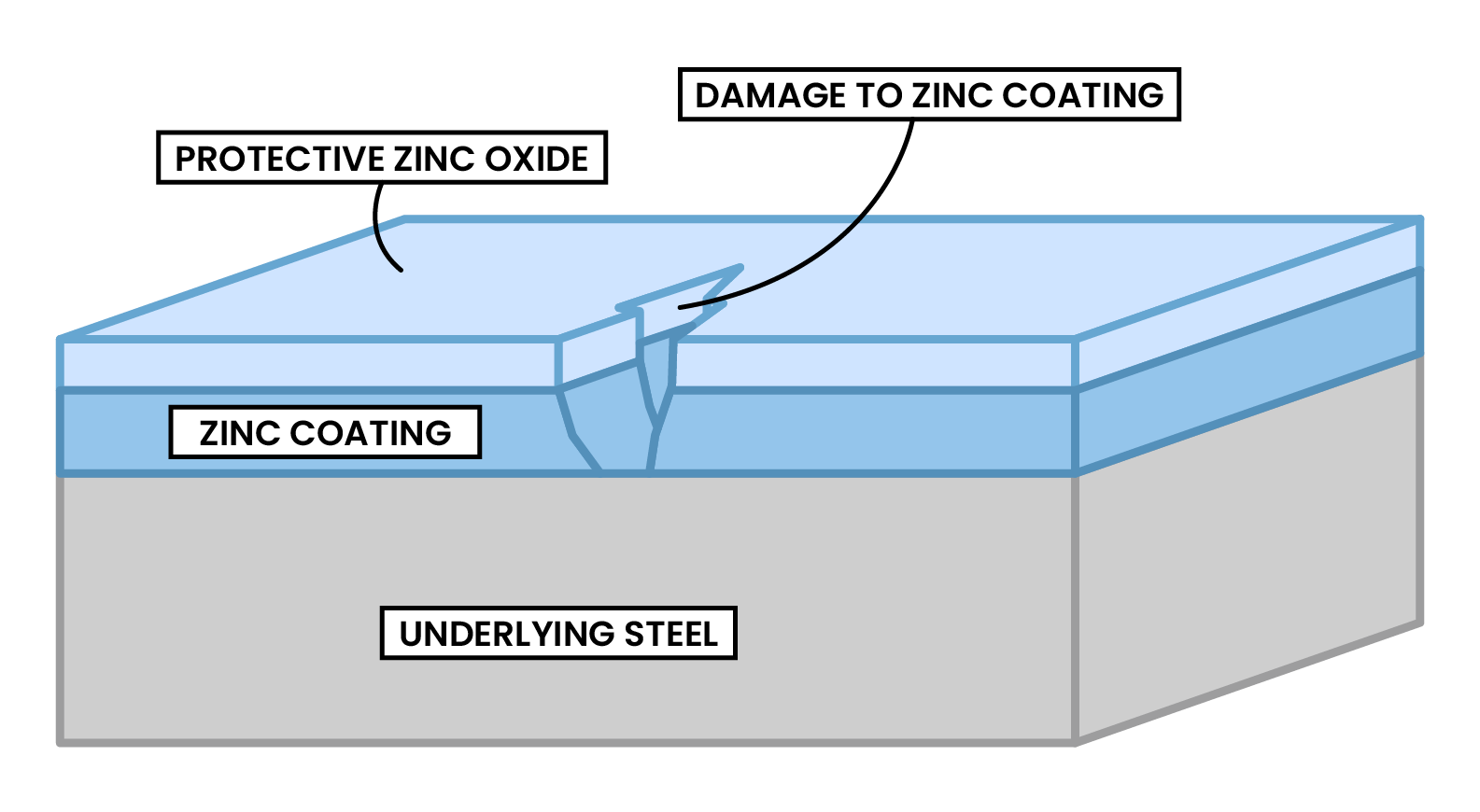
METHOD 2 GALVANISING
- Rusting can be prevented by using metals higher in reactivity than iron
- Galvanising: iron is protected by coating with a layer of zinc
- Zn reacts with oxygen and carbon dioxide in the air, which protects the iron
- Zn loses electrons more readily than Fe
- Fe stays protected because it accepts the electrons released by Zn, so Fe does not undergo oxidation
- If the coating is damaged or scratched is still protected from rusting because Zn preferentially corrodes as it is higher up in the reactivity series than iron
METHOD 3 SACRIFICIAL CORROSION
- Sacrificial corrosion occurs when a more reactive metal is intentionally allowed to corrode
Example: ships’ hull
- ships’ hulls sometimes have large blocks of magnesium attached
- The blocks slowly corrode and provide protection to the hull in the same way the zinc does
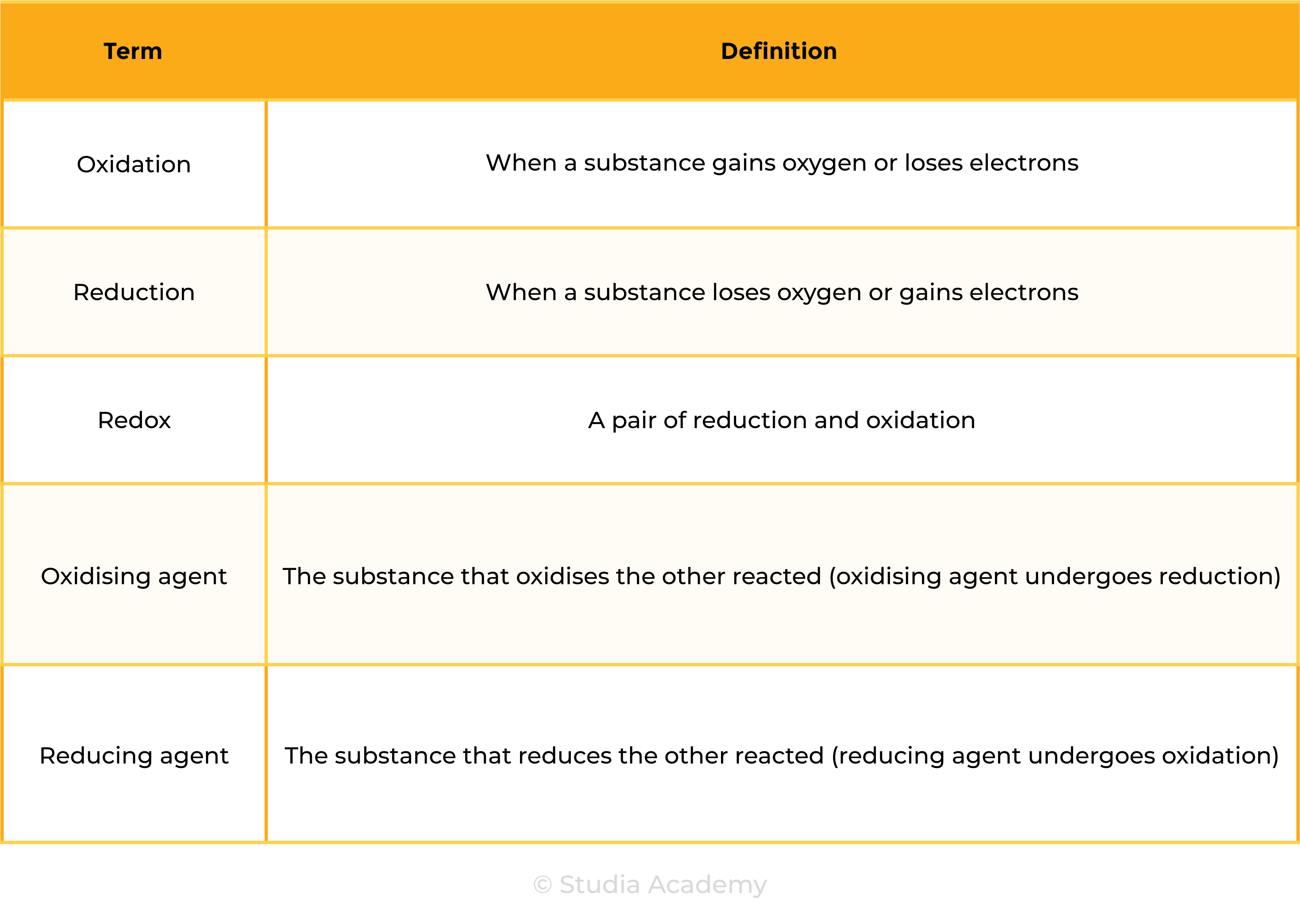
2.4.6 Understand the terms:
- Oxidation
- Reduction
- Redox
- Oxidising agent
- Reducing agent
in terms of gain or loss of oxygen and loss or gain of electrons.
REDOX REACTION (IN TERMS OF OXYGEN)
- Zn + CuO → ZnO + Cu
- This displacement reaction can be classified as a redox reaction
- Oxidation occurs together with reduction simultaneously
- CuO loses oxygen, so it undergoes reduction
- Zn gains oxygen, so it undergoes oxidation
- CuO oxidises Zn, so it is an oxidising agent
- Zn reduces CuO, so it is an reducing agent

REDOX REACTION (IN TERMS OF ELECTRON)
- Displacement reaction can also be analysed in terms of electron transfer
- An ionic equation is used to help better understand the electron transfer
- Zn + CuO → ZnO + Cu
- Ionic equation: Zn + Cu2+ + O2- → Zn2+ + O2- + Cu
- O2- is the spectator ion that appears on both sides, it is removed for the net ionic equation
- Net ionic equation: Zn + Cu2+ → Zn2+ + Cu
- The equation can be further split into two half equations that show oxidation and reduction individually:
Zn → Zn2+ + 2e–
Cu2++ 2e– → Cu
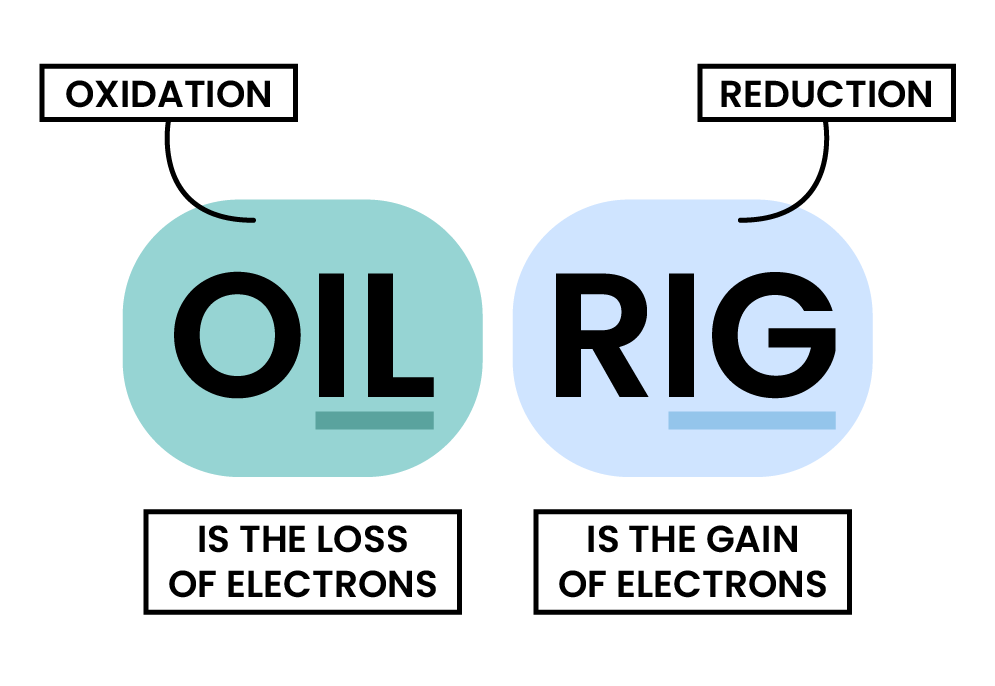
- Zn loses electrons, so it undergoes oxidation
- Cu2+ gains electrons, so it undergoes reduction
2.4.7 Practical: investigate reactions between dilute hydrochloric and sulfuric acids and metals (e.g. magnesium, zinc and iron)
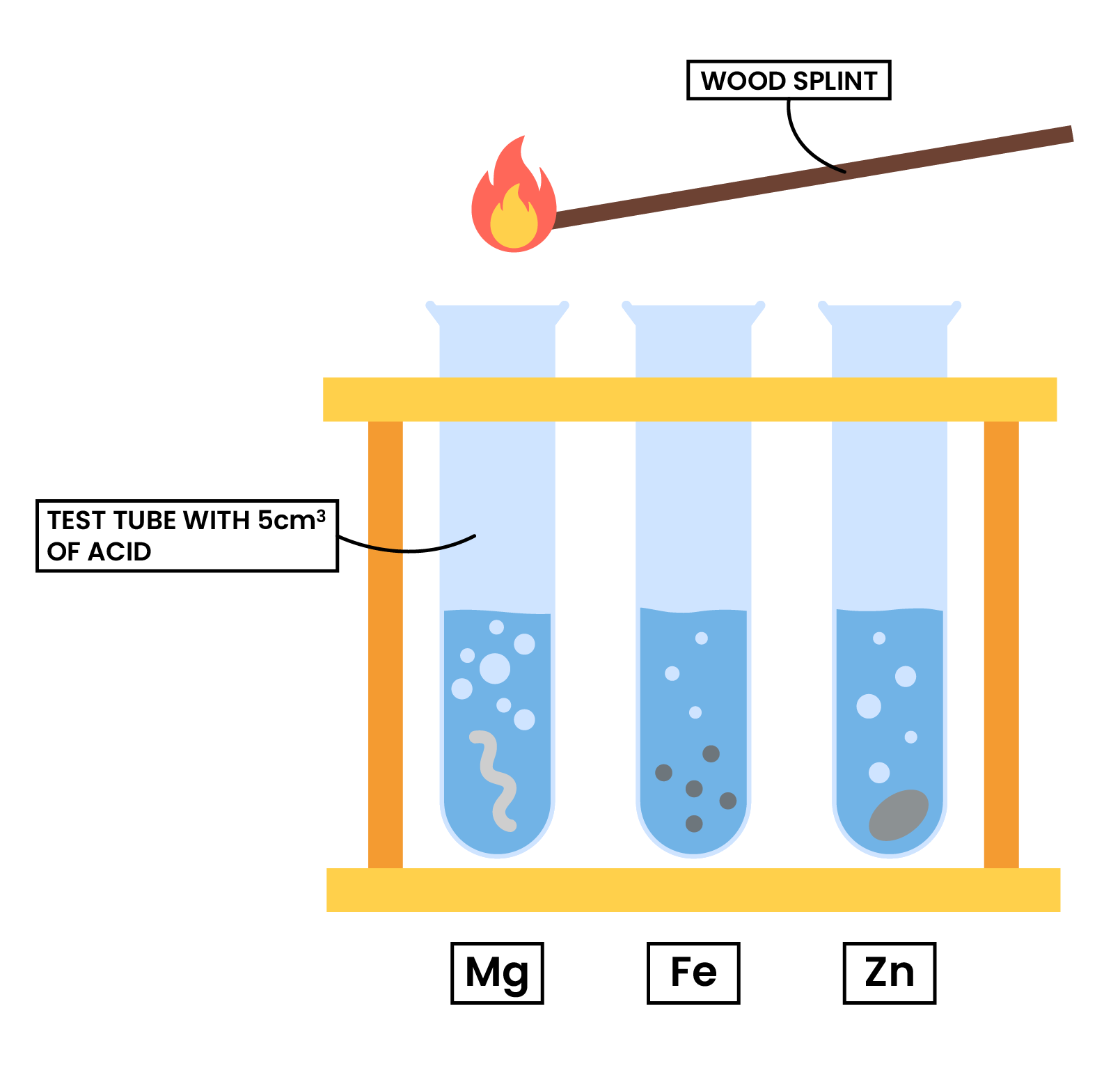
METHODS
- Use a measuring cylinder to measure 5cm3 of dilute hydrochloric acid
- Add same mass of magnesium ribbon, iron filings and zinc turnings to different test tubes
- Observe what happens
- Use a light splint to test for any gases given off
- Repeat the experiment with dilute sulfuric acid
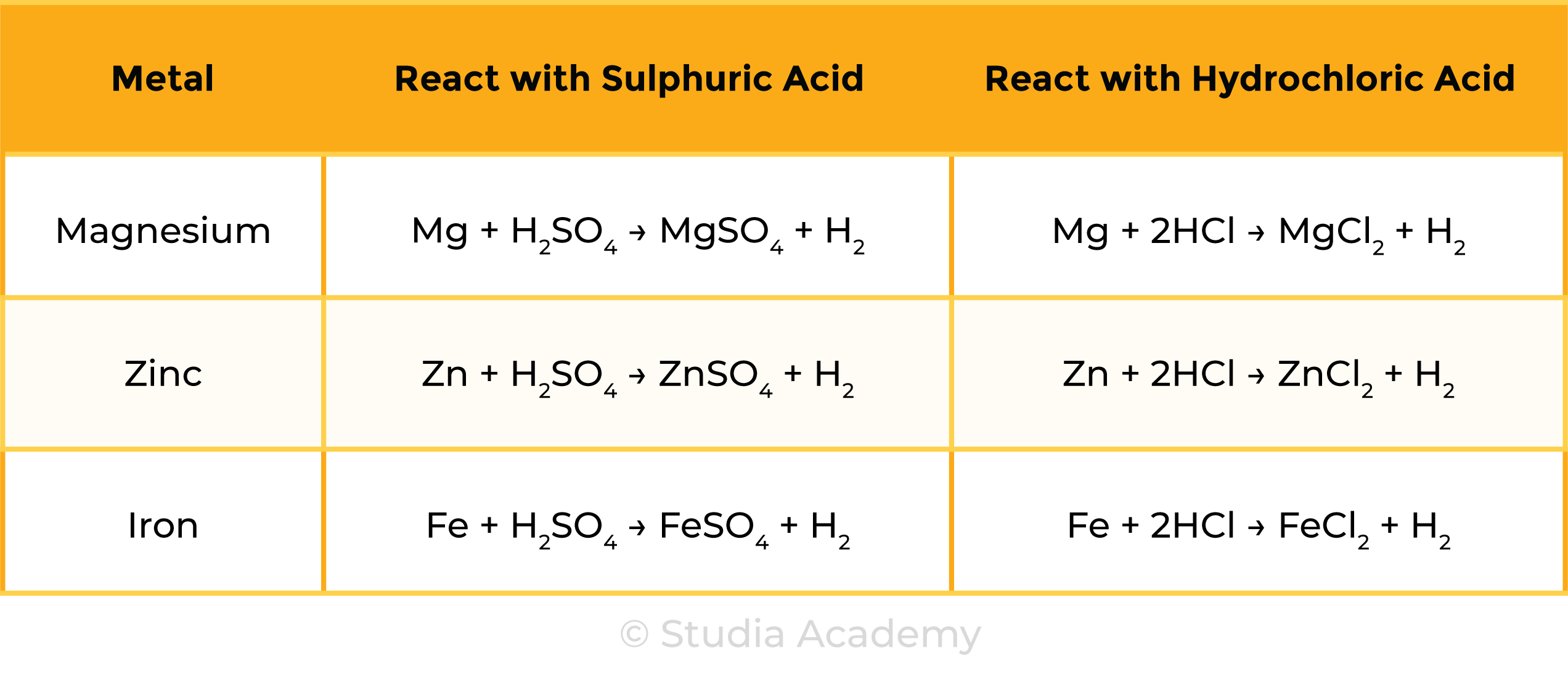
Observations
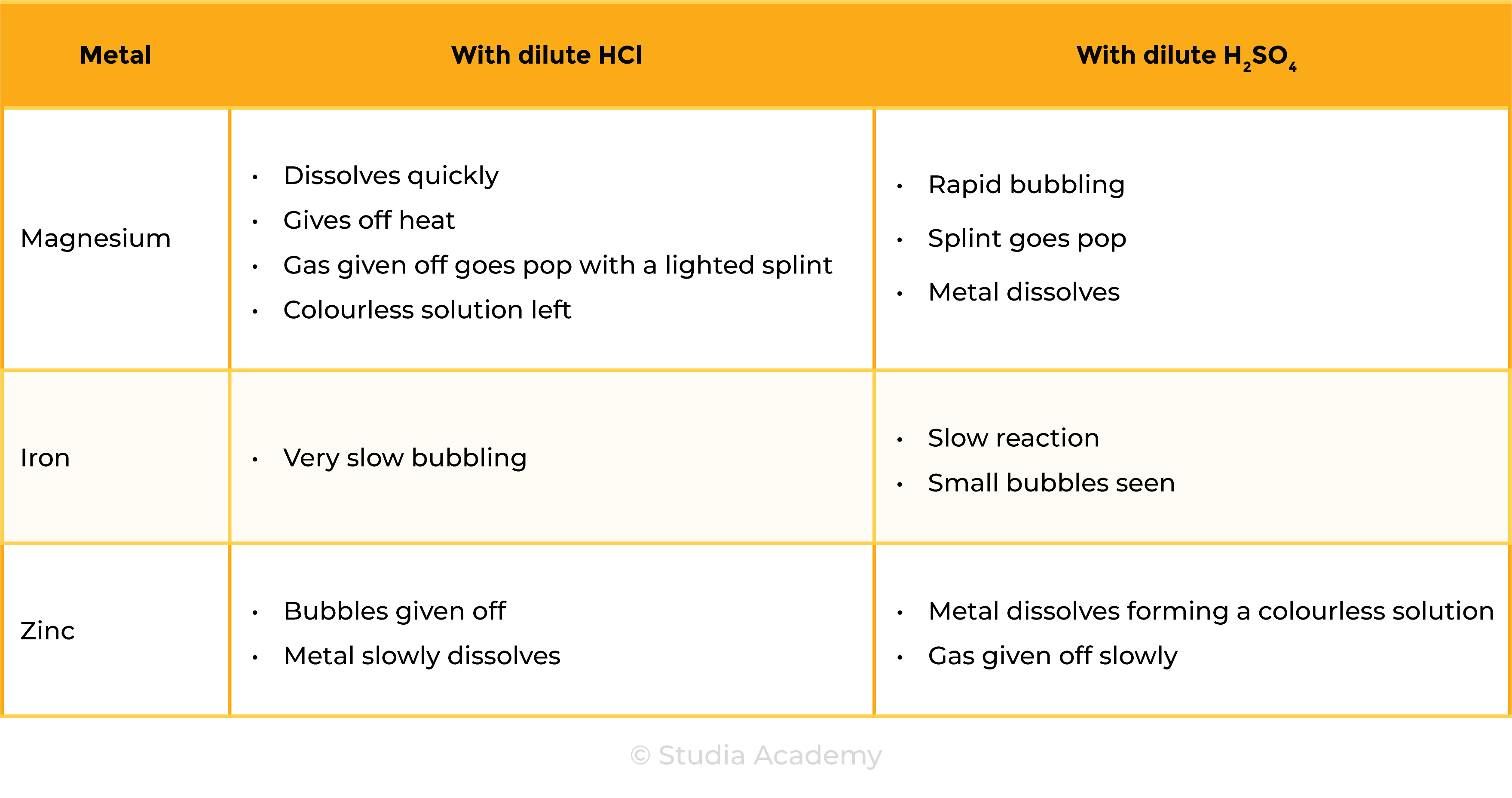
Reactions
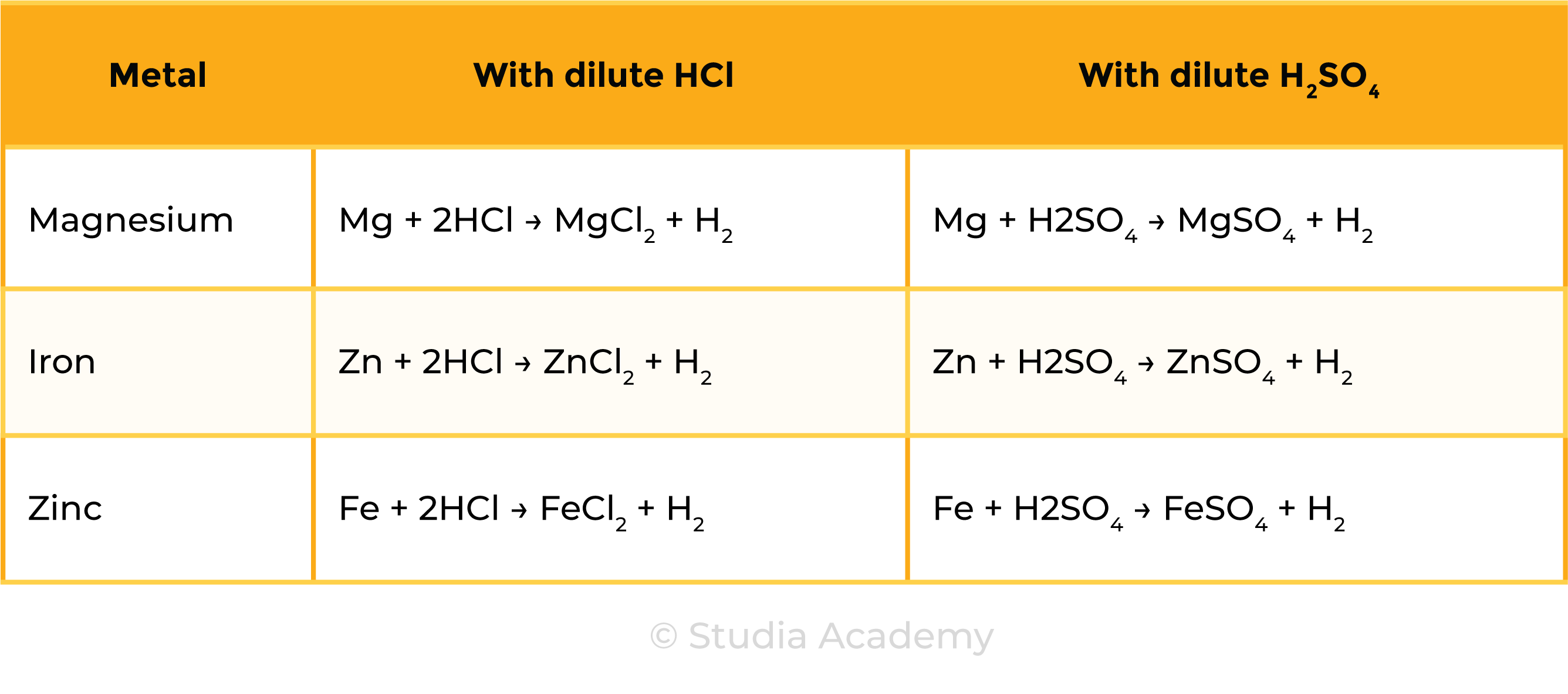
CONCLUSION
- The metals can be ranked in reactivity order Mg > Zn > Fe
- The three metals react in the same with both acids
- Hydrogen and a metal salt solution is produced

