REVISION NOTES
5.2.1P Explain why heating a system will change the energy stored within the system and raise its temperature or produce changes of state
5.2.2P Describe the changes that occur when a solid melts to form a liquid, and when a liquid evaporates or boils to form a gas

5.2.3P Describe the arrangement and motion of particles in solids, liquids and gases

5.2.4P Practical: obtain a temperature–time graph to show the constant temperature during a change of state
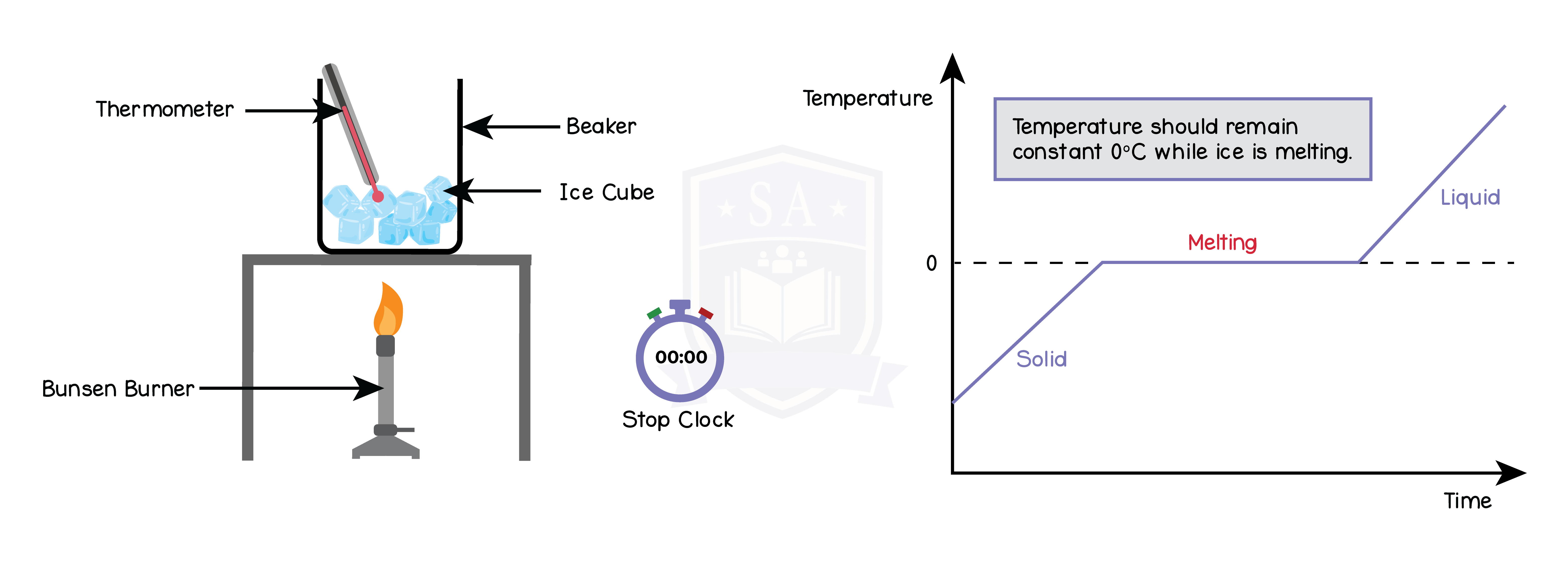
5.2.5P Know that specific heat capacity is the energy required to change the temperature of an object by one degree Celsius per kilogram of mass (J/kg °C)
5.2.6P Use the equation:
change in thermal energy = mass × specific heat capacity × change in temperature

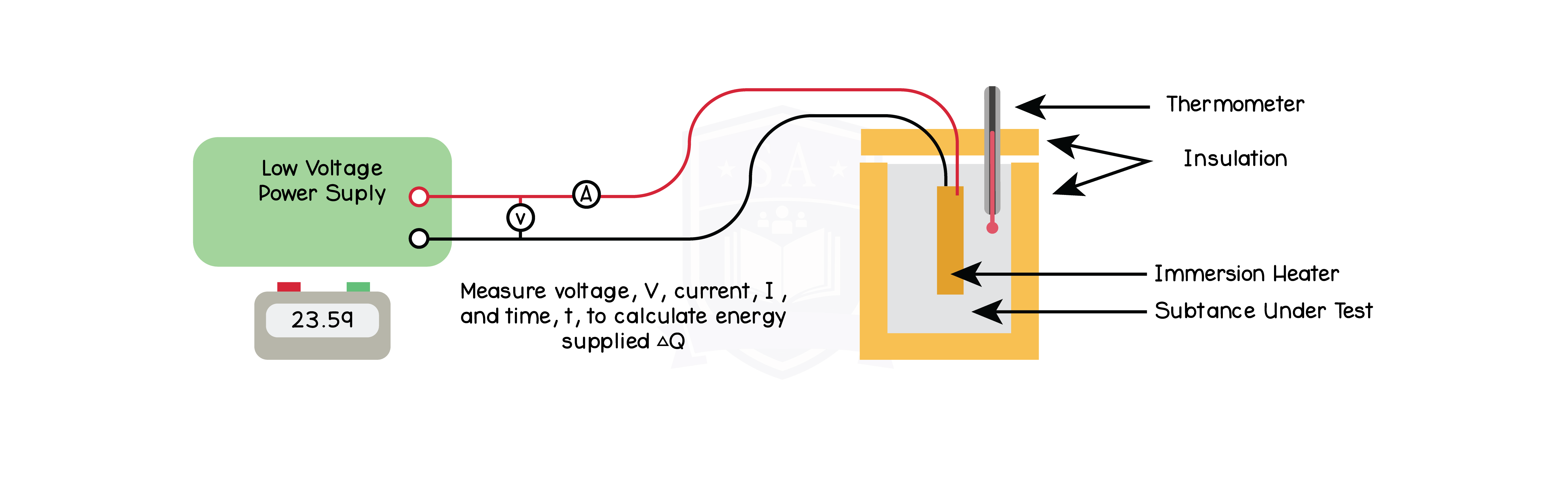
5.2.7P Practical: investigate the specific heat capacity of materials including water and some solids

© 2025 Studia Academy. All rights reserved.