REVISION NOTES
3.3.1 Know that some reactions are reversible and this is indicated by the symbol ⇌ in equations
COMPLETE REACTION
REVERSIBLE REACTION
3.3.2 Describe reversible reactions such as the dehydration of hydrated copper(II) sulfate and the effect of heat on ammonium chloride
Examples of Reversible Reaction
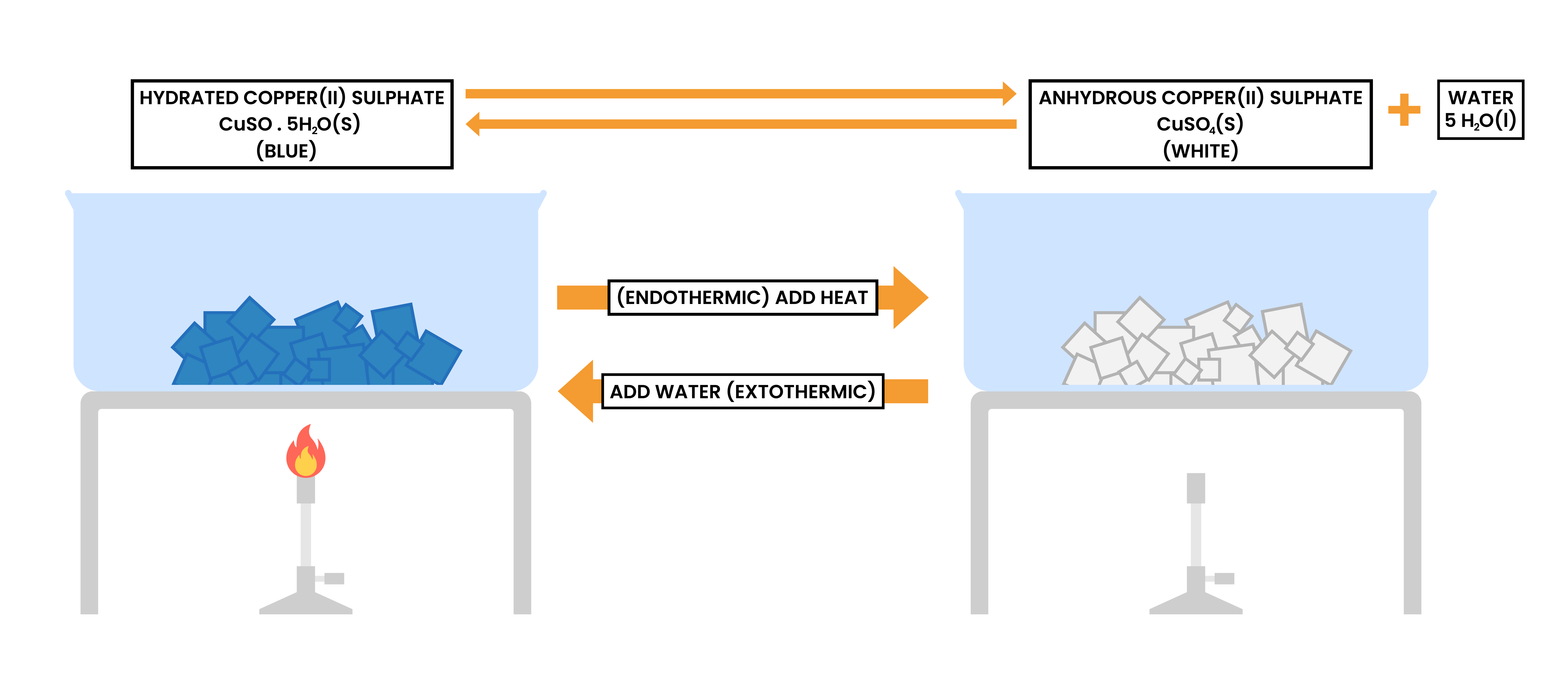
Example 1 Dehydration of Hydrated Copper(II) Sulphate
CuSO4・5H2O (s) ⇌ CuSO4 (s) + 5H2O (l)
EXAMPLE 2 THERMAL DECOMPOSITION OF AMMONIUM CHLORIDE
NH4Cl (s) → NH3 (g) + HCl (g)
NH3 (g) + HCl (g) → NH4Cl (s)
ammonium chloride ⇌ ammonia + hydrogen chloride
NH4Cl (s) ⇌ NH3 (g) + HCl (g)
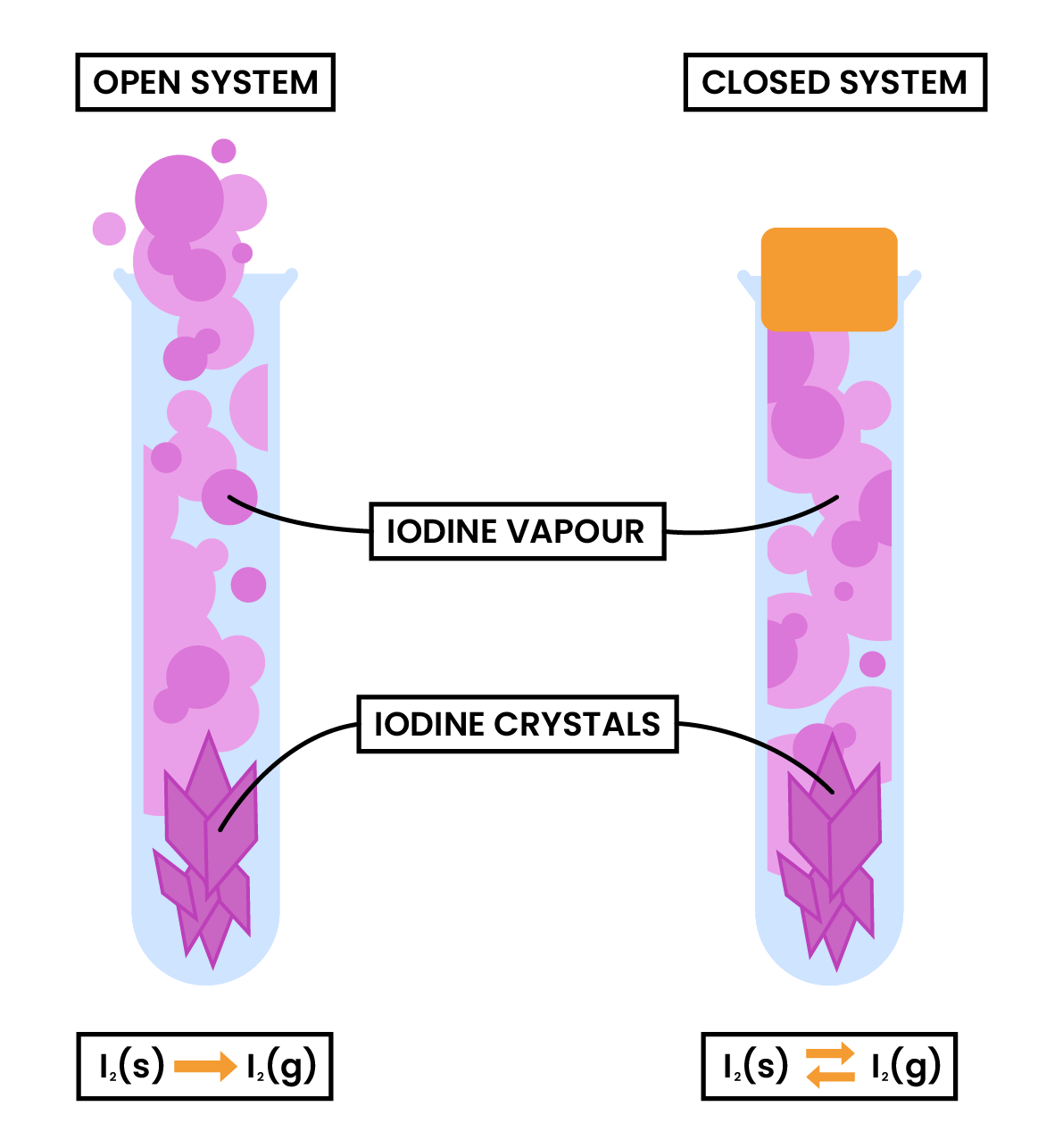
3.3.3C Know that a reversible reaction can reach dynamic equilibrium in a sealed container
DYNAMIC EQUILIBRIUM
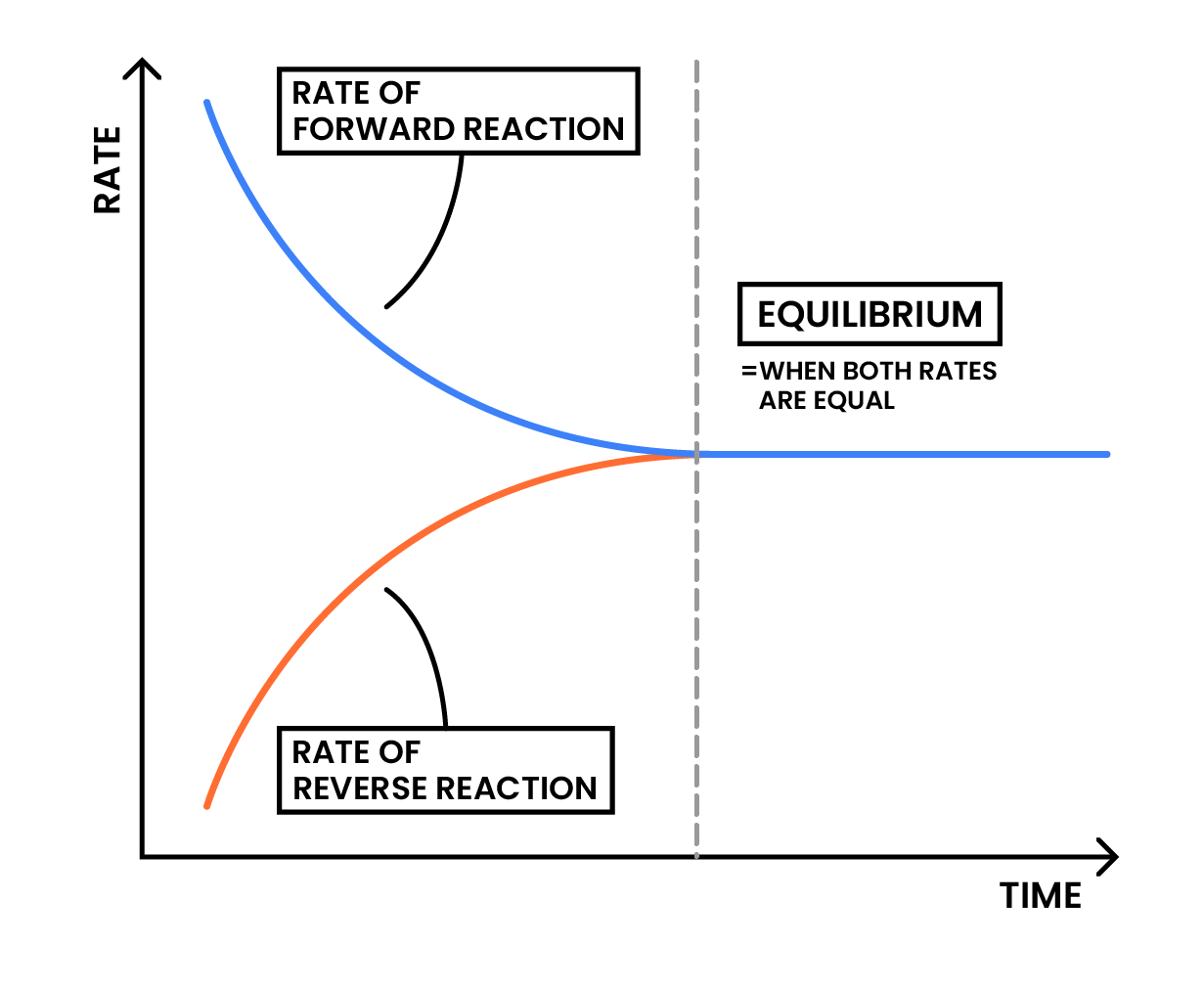
3.3.4C Know that the characteristics of a reaction at dynamic equilibrium are:
When a reaction is at dynamic equilibrium:
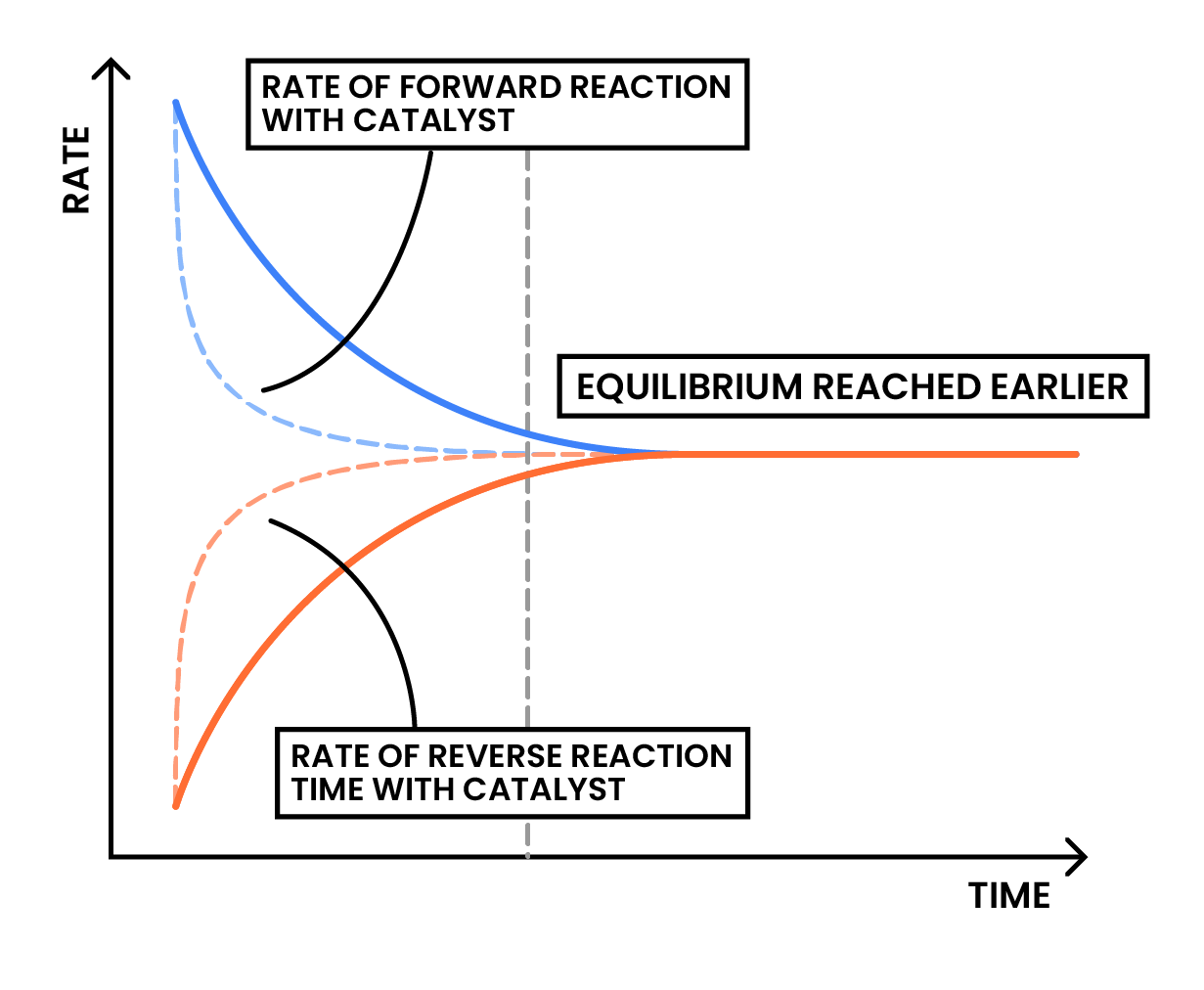
3.3.5C Understand why a catalyst does not affect the position of equilibrium in a reversible reaction
Presence of catalyst does not affect the position of equilibrium
3.3.6C Know the effect of changing either temperature or pressure on the position of equilibrium in a reversible reaction:
References to Le Chatelier’s principle are not required
Effect of Temperature
Example
ICl + Cl2 ⇌ ICl3
Dark brown Yellow
When the equilibrium mixture is heated, it becomes dark brown in colour.
HOW DO WE KNOW WHETHER THE BACKWARD REACTION IS EXOTHERMIC OR ENDOTHERMIC?
Effect of Pressure
Example
2NO2 ⇌ N2O4
Brown gas Colourless gas
WHAT IS THE EFFECT OF AN INCREASE IN PRESSURE ON THE POSITION OF EQUILIBRIUM?

© 2025 Studia Academy. All rights reserved.