REVISION NOTES
3.1.1 Know that chemical reactions in which heat energy is given out are described as exothermic, and those in which heat energy is taken in are described as endothermic

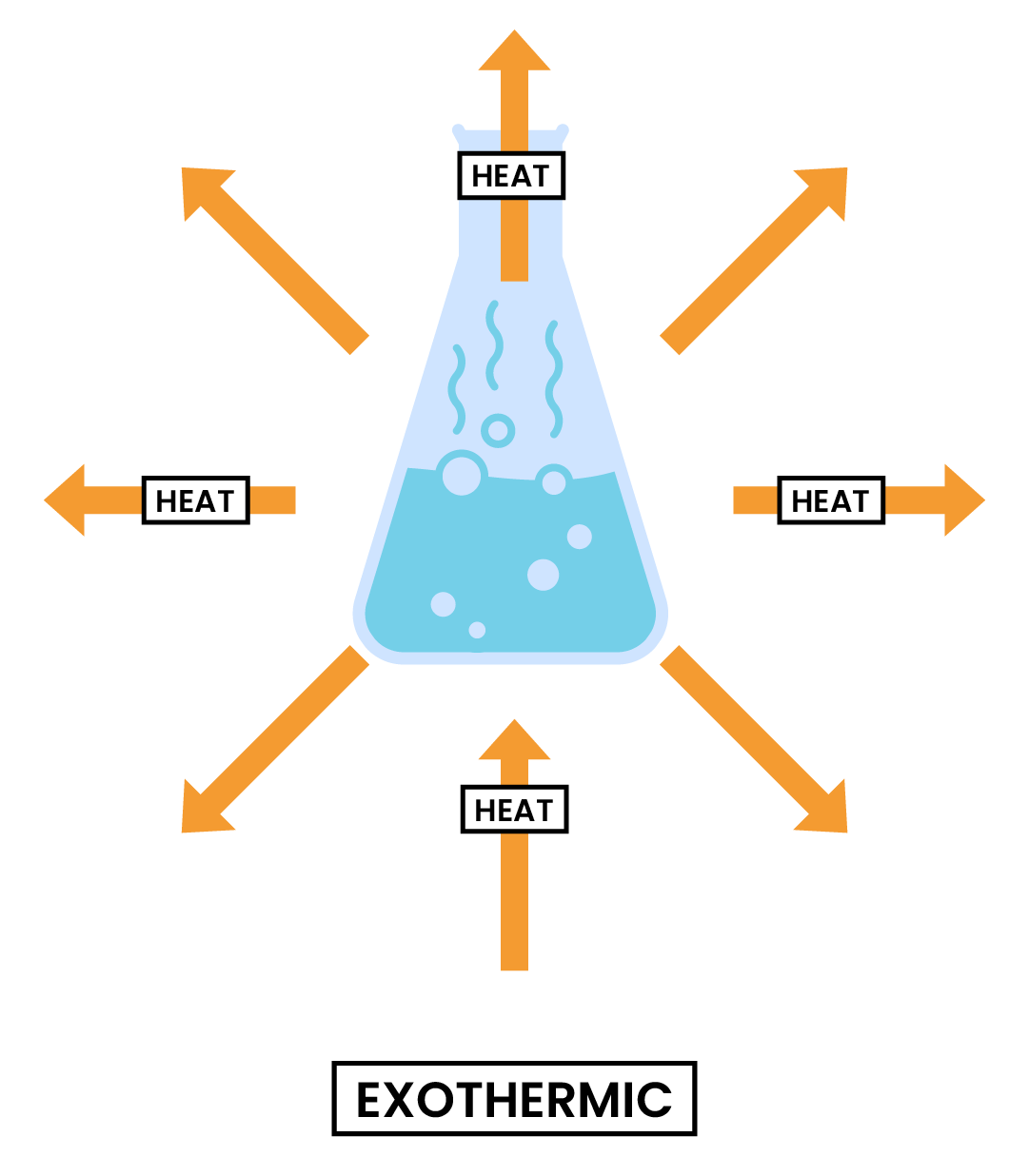
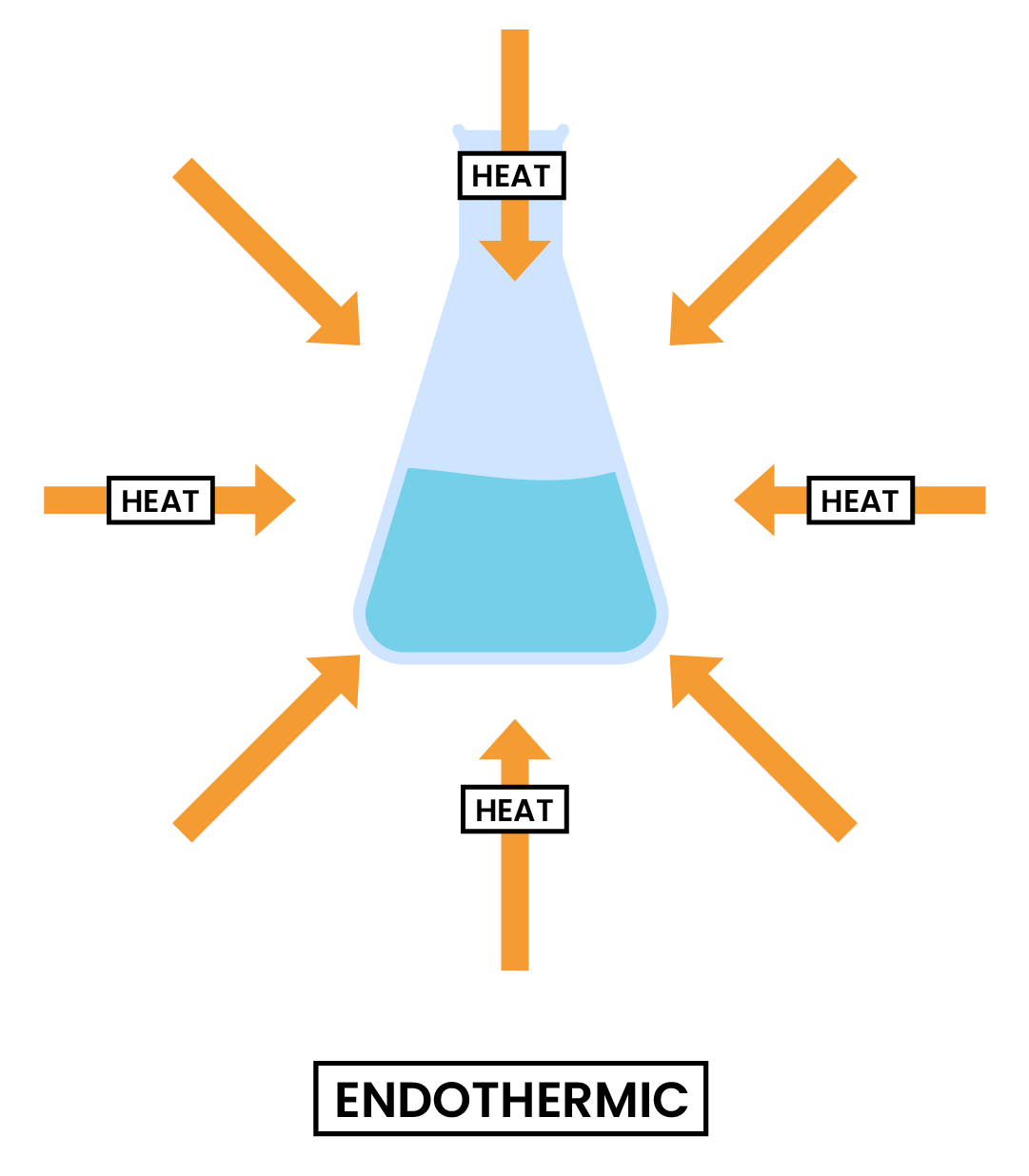
3.1.2 Describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation
CALORIMETRY
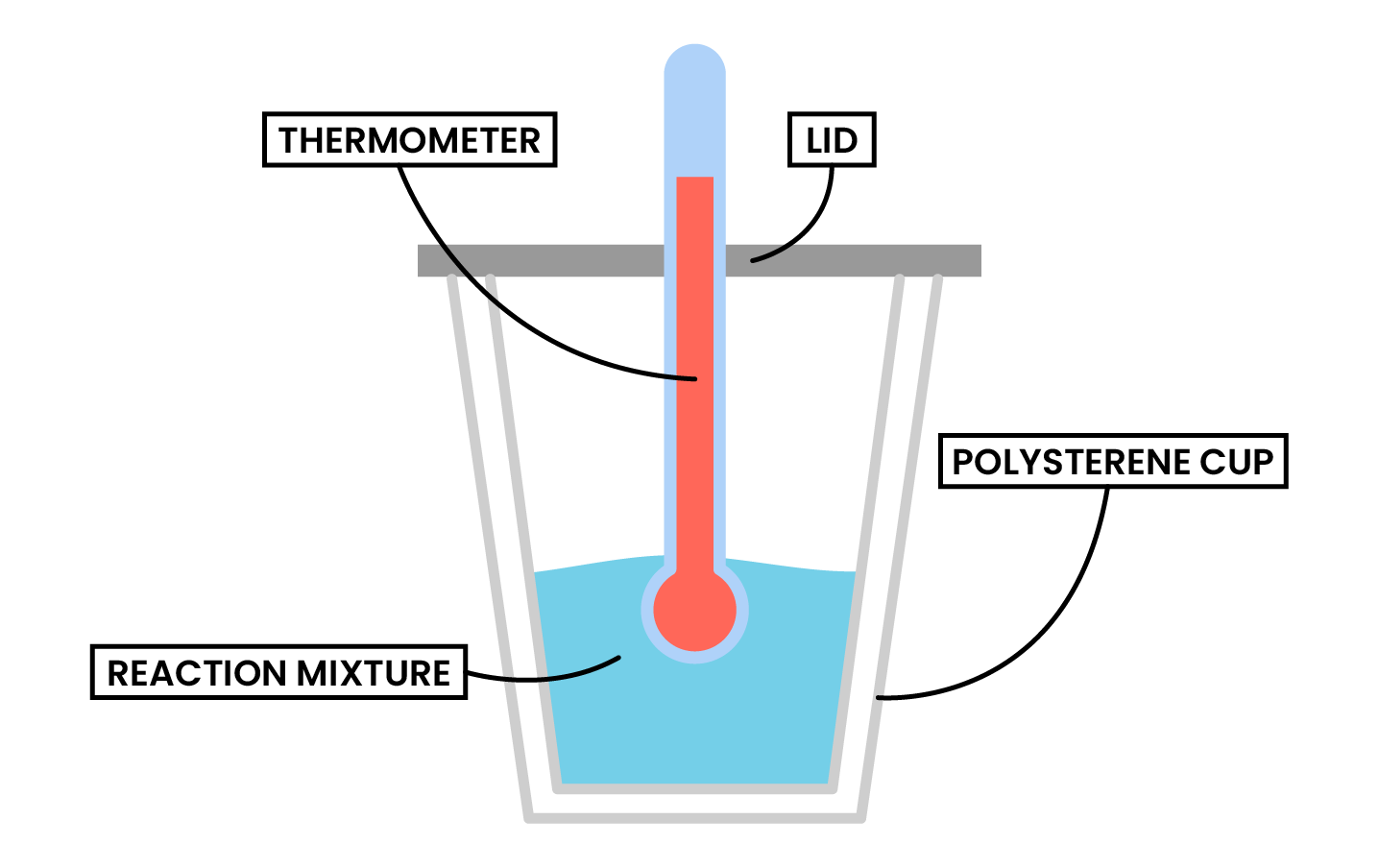
TYPE 1 REACTIONS IN SOLUTION
METHODS
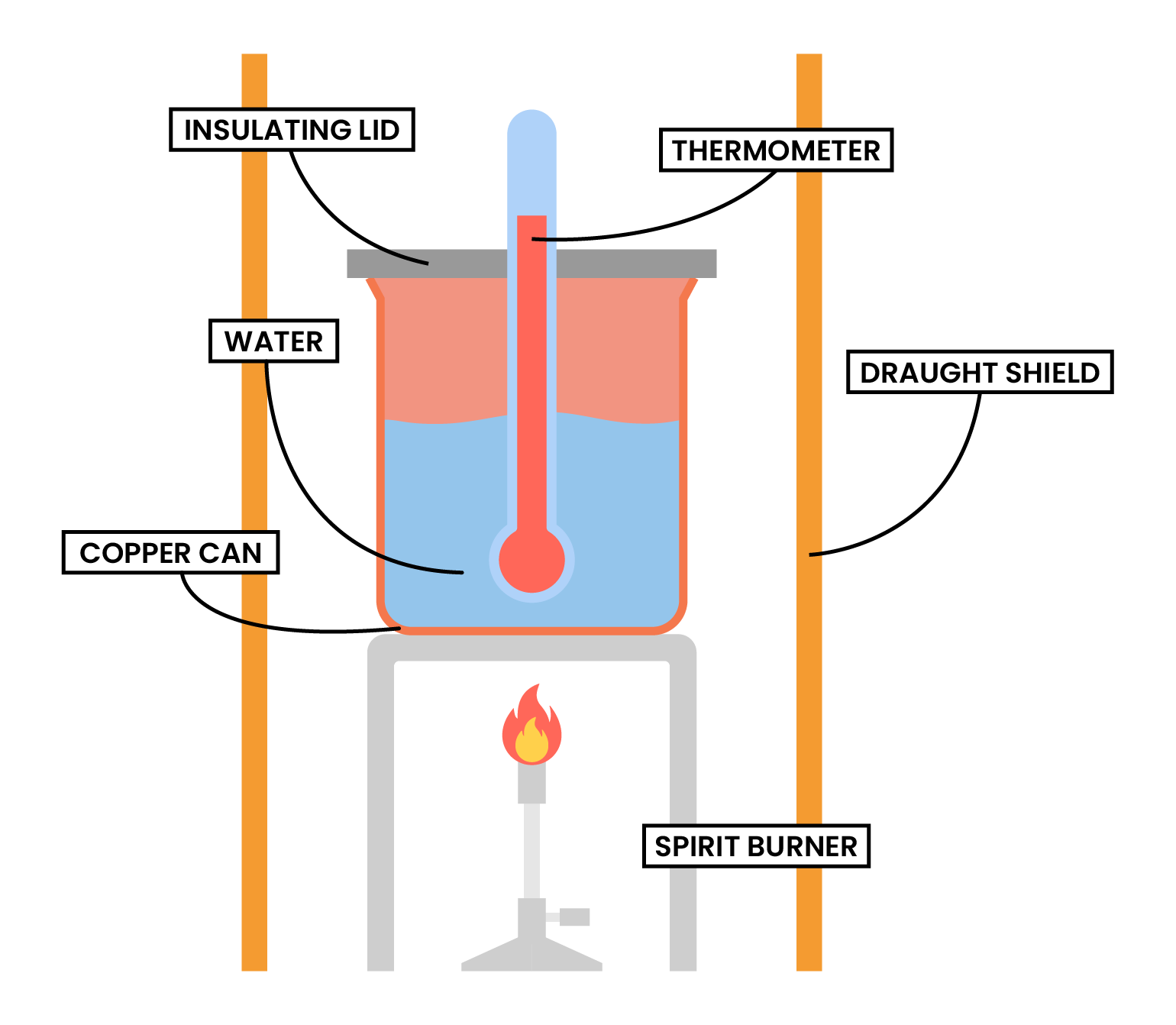
TYPE 2 COMBUSTION
METHODS
KEY POINTS
3.1.3 Calculate the heat energy change from a measured temperature change using the expression Q = mcΔT
SPECIFIC HEAT CAPACITY (c)
SPECIFIC HEAT CAPACITY (c)
Energy transferred as heat can be calculated by:
q = m ✕ c ✕ ΔT
q = heat transferred (J)
m = mass of water (g)
c = specific heat capacity (J g–1 K–1)
ΔT = temperature change (K)
KEY POINTS
ΔT = final temperature – initial temperature
= 58oC – 24oC
= 34oC
q = m ✕ c ✕ ΔT
= 300 ✕ 4.18 ✕ 34
= 42636 J
= 42.636 kJ
3.1.4 Calculate the molar enthalpy change (ΔH) from the heat energy change, Q
MOLAR ENTHALPY CHANGE
CALCULATION OF ΔH [TITLE]
Molar enthalpy change = heat change for the reaction ÷ number of moles
ΔH = – q ÷ n
EXAMPLE (CONT.)
2.087 g of ethanol (Mr = 46) was burned in an alcohol burner and used to heat 300 g of water in a calorimeter. The initial and final temperature of water was 24oC and 58oC respectively. Calculate the molar enthalpy of combustion.
q = m ✕ c ✕ ΔT
= 42.636 kJ
(from 3.3)
n = mass (ethanol) ÷ molar mass (ethanol)
= 2.087 ÷ 46
= 0.0454 mol
ΔH = – q ÷ n
= – 42.636 ÷ 0.0454
= – 939 kJ per mol
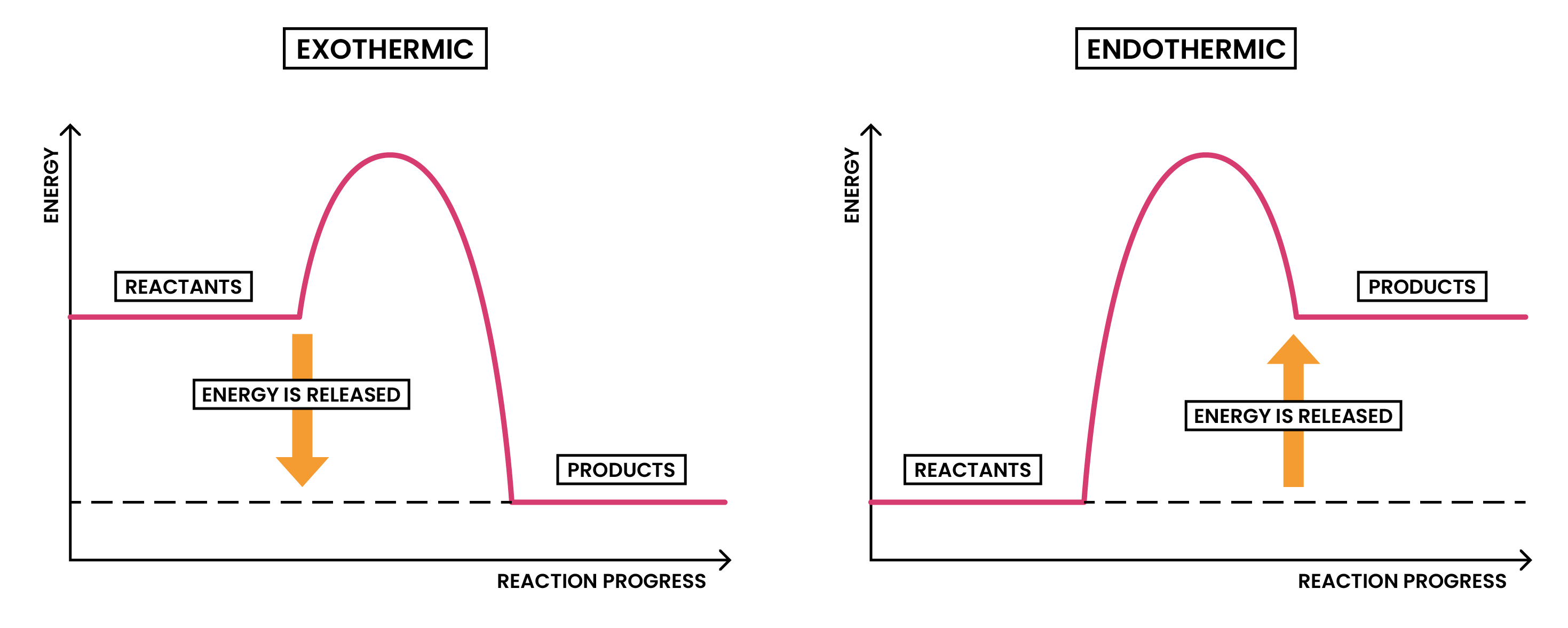
3.1.5C Draw and explain energy level diagrams to represent exothermic and endothermic reactions
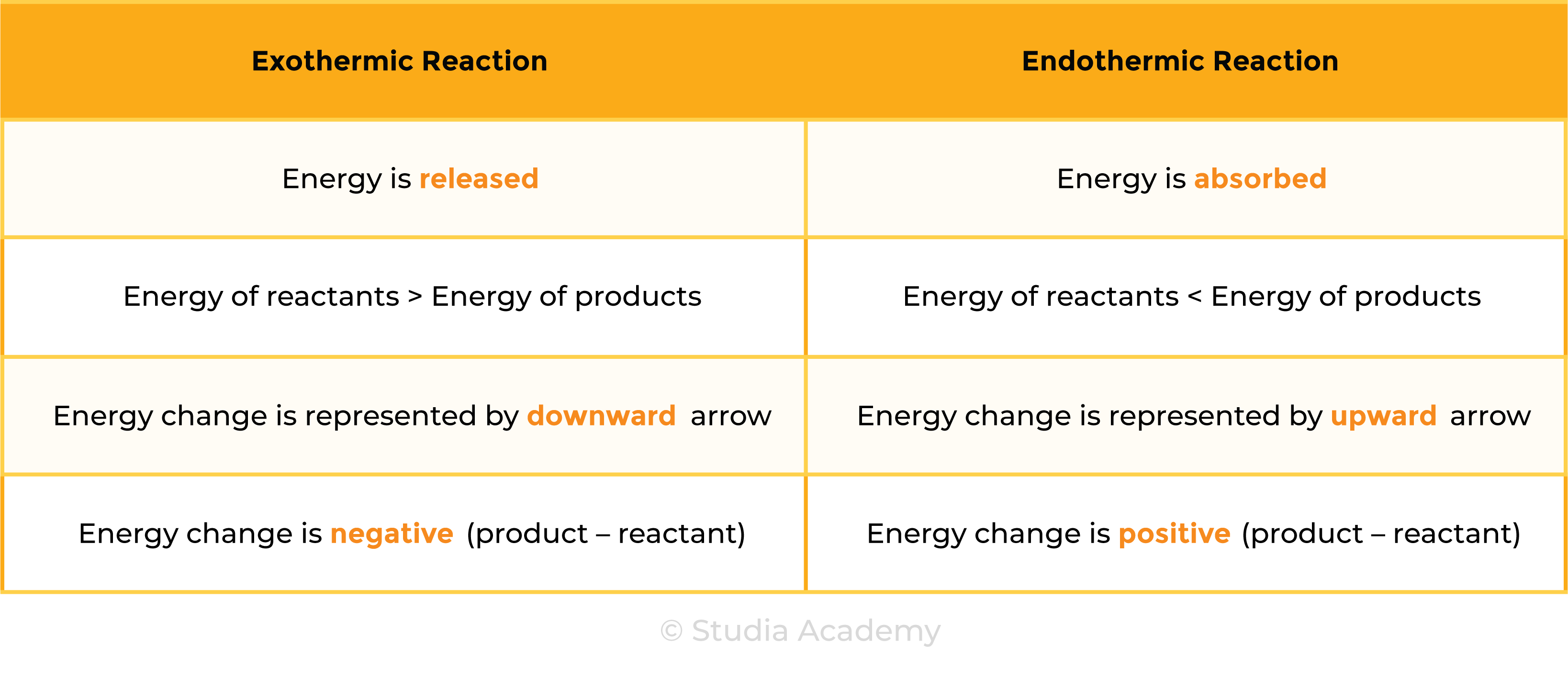
ENERGY LEVEL DIAGRAMS
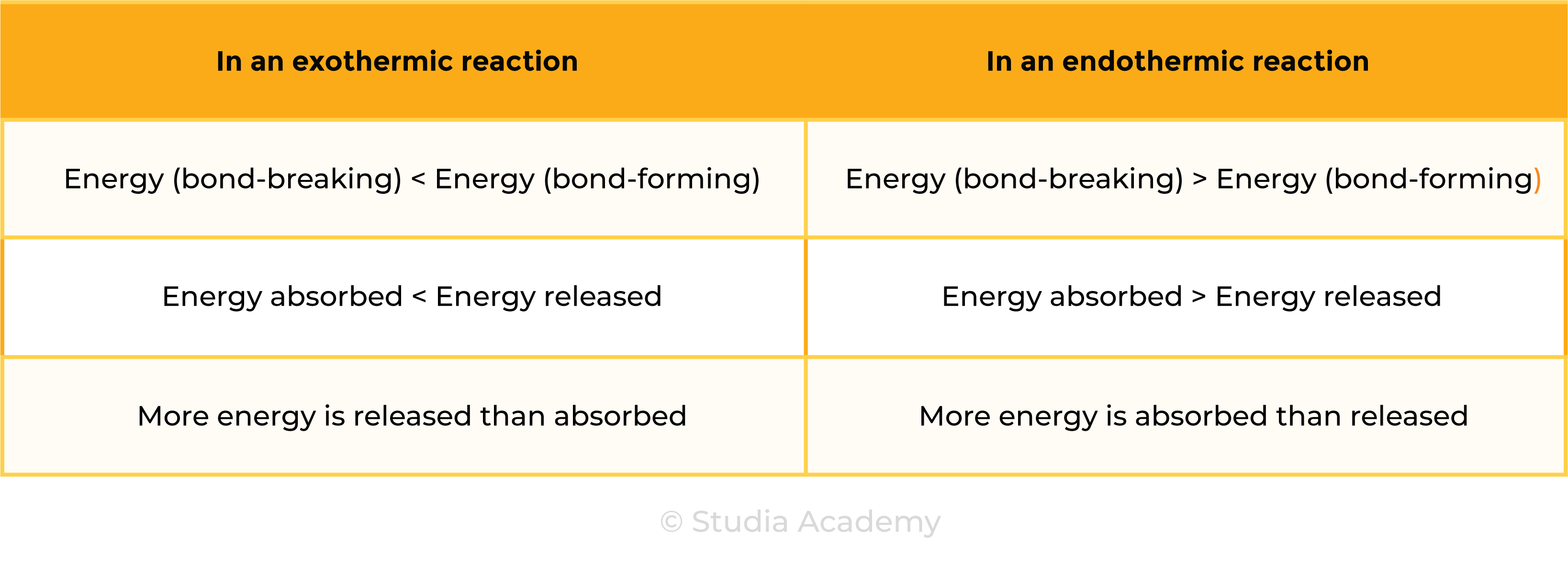
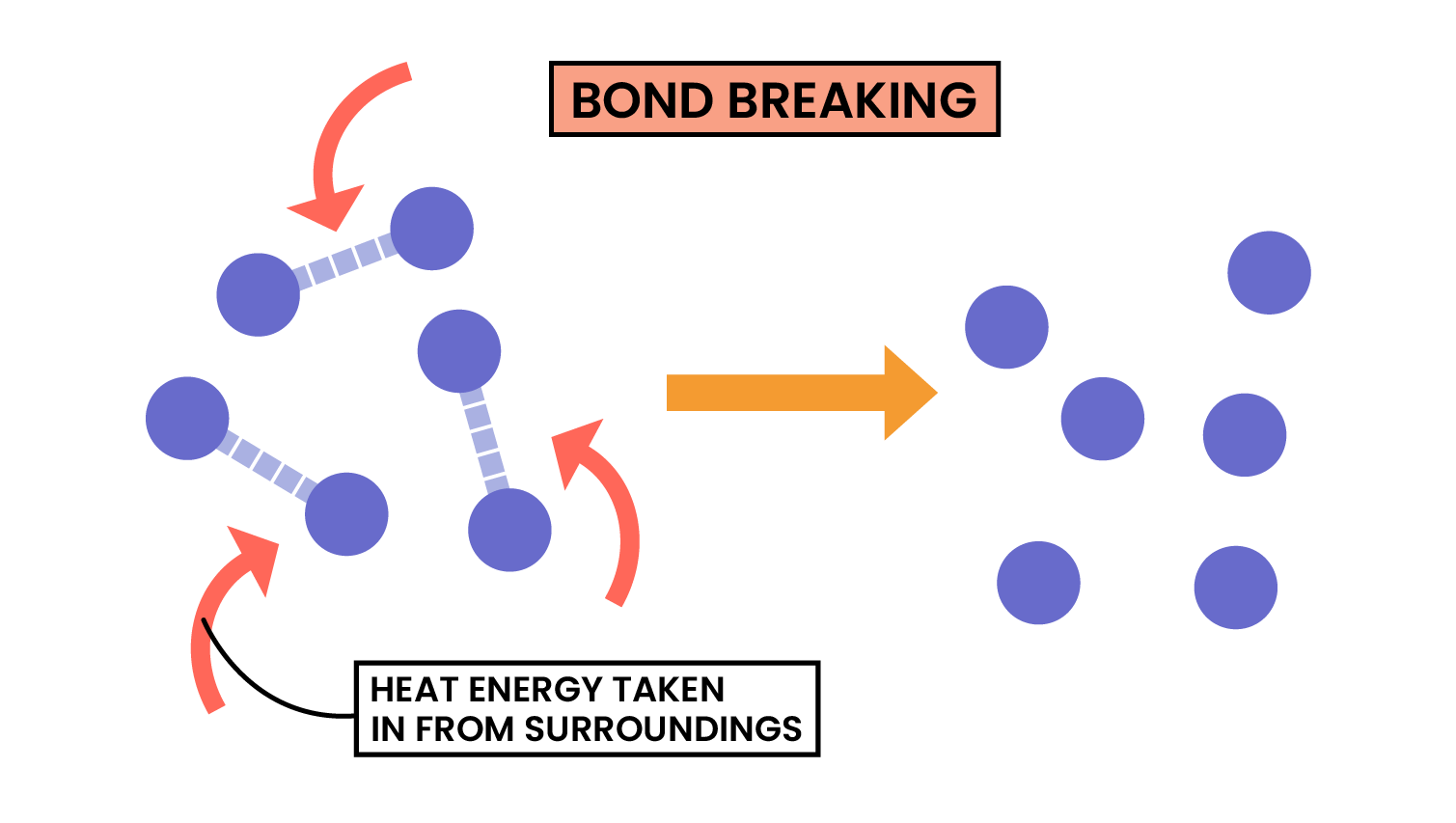
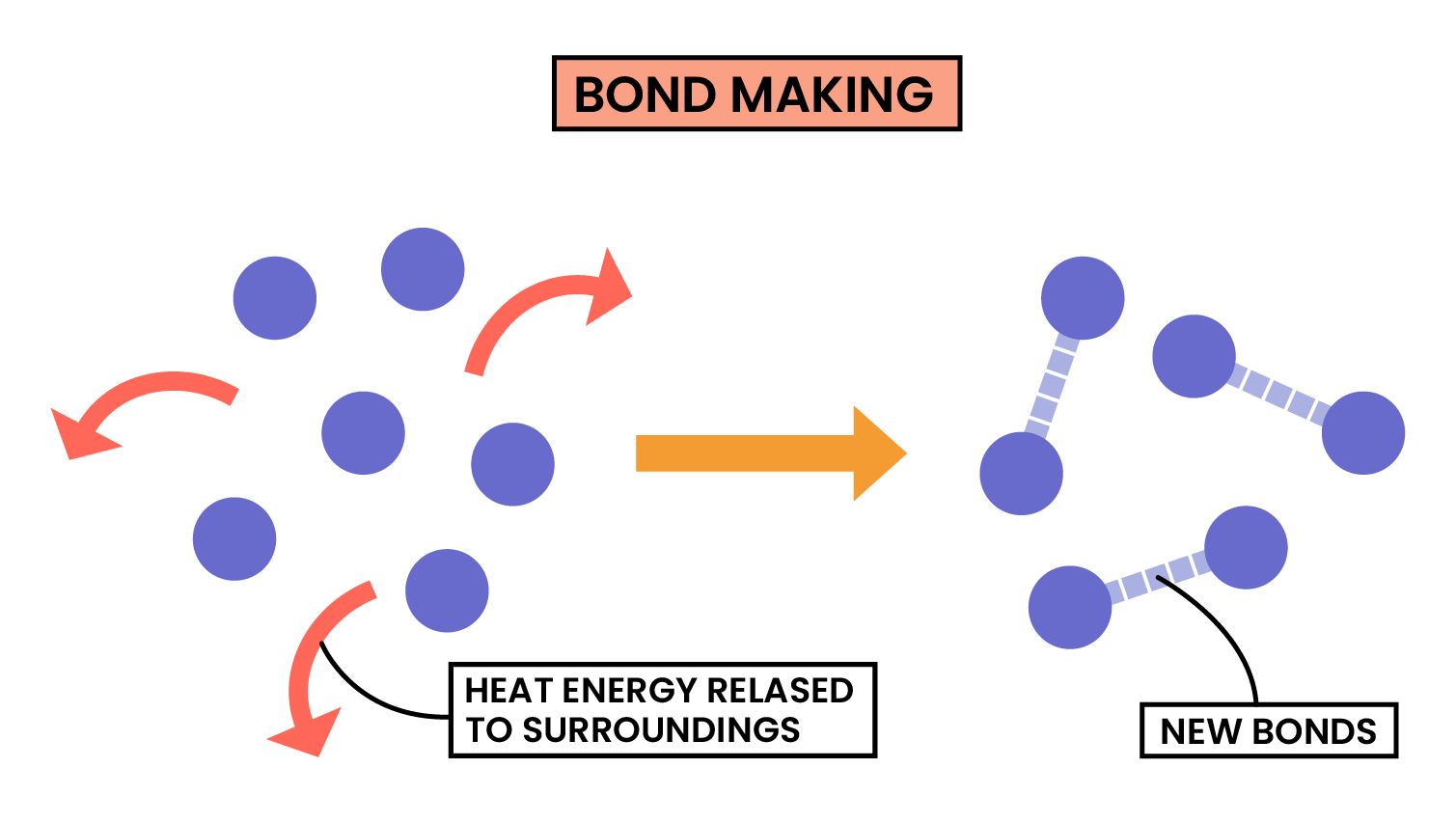
3.1.6C Know that bond-breaking is an endothermic process and that bond-making is an exothermic process
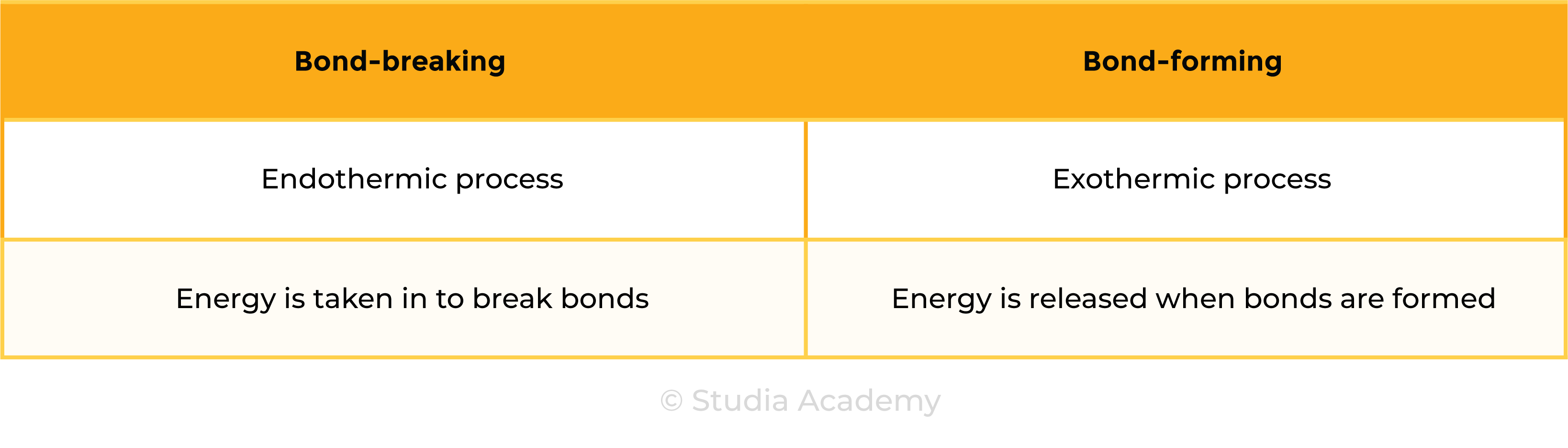
Use the idea of magnets:
Pulling apart magnets or bond breaking, requires energy, so is endothermic.
Pushing magnets together or bond making, does not require energy (releases energy in bond making), so is exothermic.
3.1.7C Use bond energies to calculate the enthalpy change during a chemical reaction
ENTHALPY CHANGE
Enthalpy change = Energy taken in – Energy given out
Enthalpy change = Bond Energies of Reactants – Bond Energies of Products
EXAMPLE
Calculate the enthalpy change for the following reaction, using the bond energies given:
CH4 + 2O2 → CO2 + 2H2O
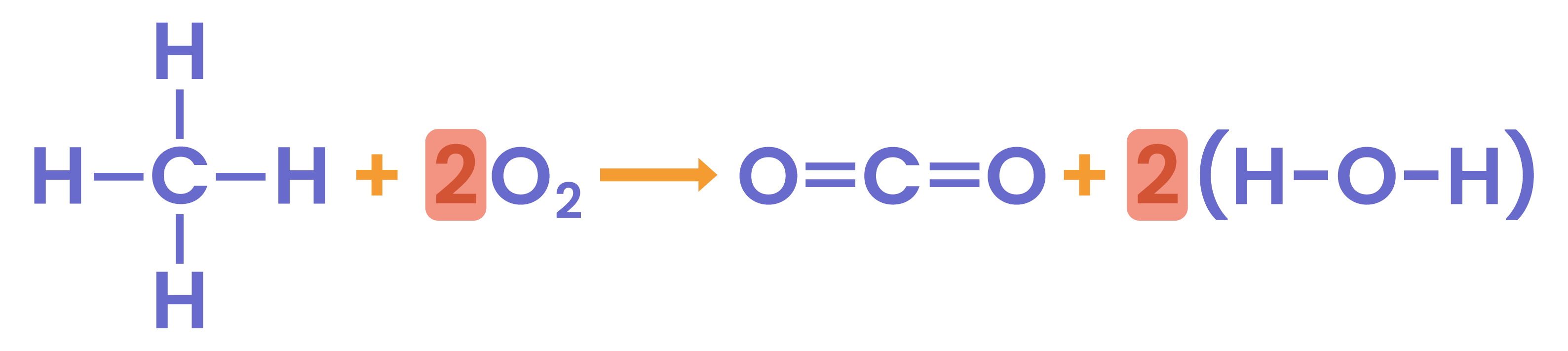
Bond energies of reactants
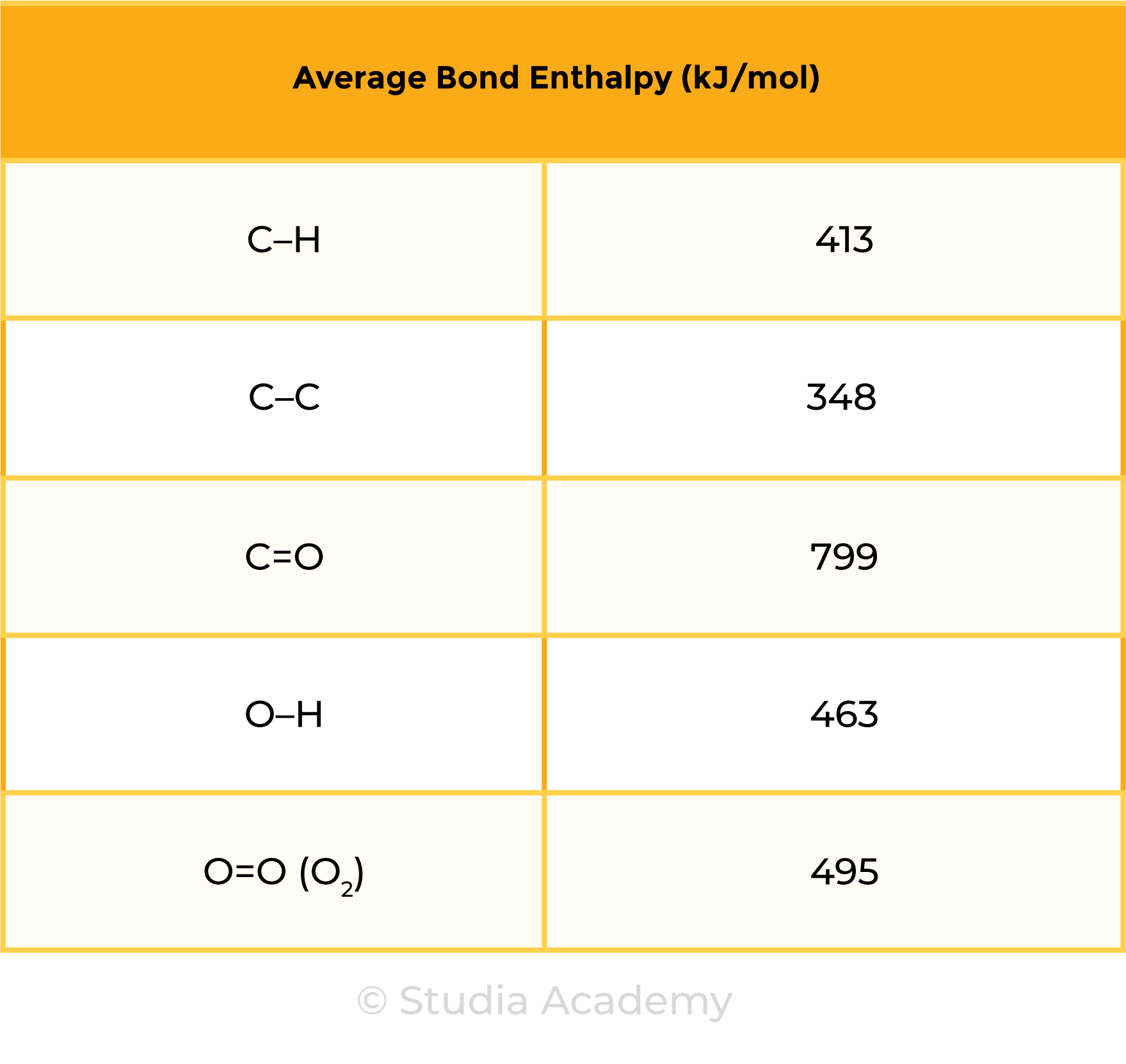
= 4 ✕ (C – H) + 2 ✕ (O = O)
= 4 ✕ 413 + 2 ✕ 495
= 2642 kJ
Bond energies of products
= 2 ✕ (C = O) + 4 ✕ (O – H)
= 2 ✕ 799 + 4 ✕ 463
= 3450 kJ
Enthalpy change
= bond energies of reactants – bond energies of products
= 2642 – 3450
= – 808 kJ/mol
3.1.8 Practical: investigate temperature changes accompanying some of the following types of change:
Type 1 Calorimetry Experiment (Dissolving, Neutralisation and Displacement)
METHODS
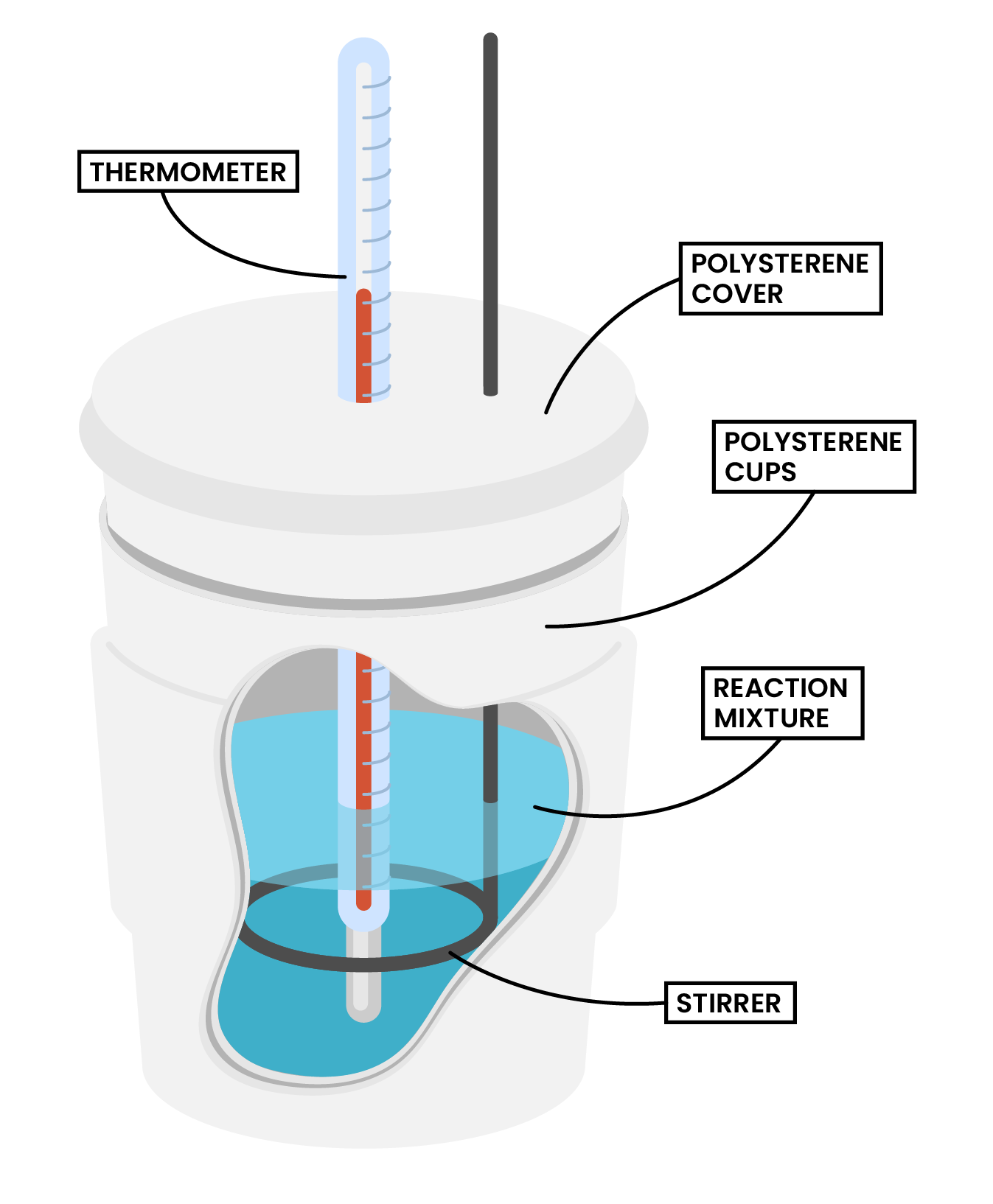
Type 2 Calorimetry Experiment (Combustion)
METHODS
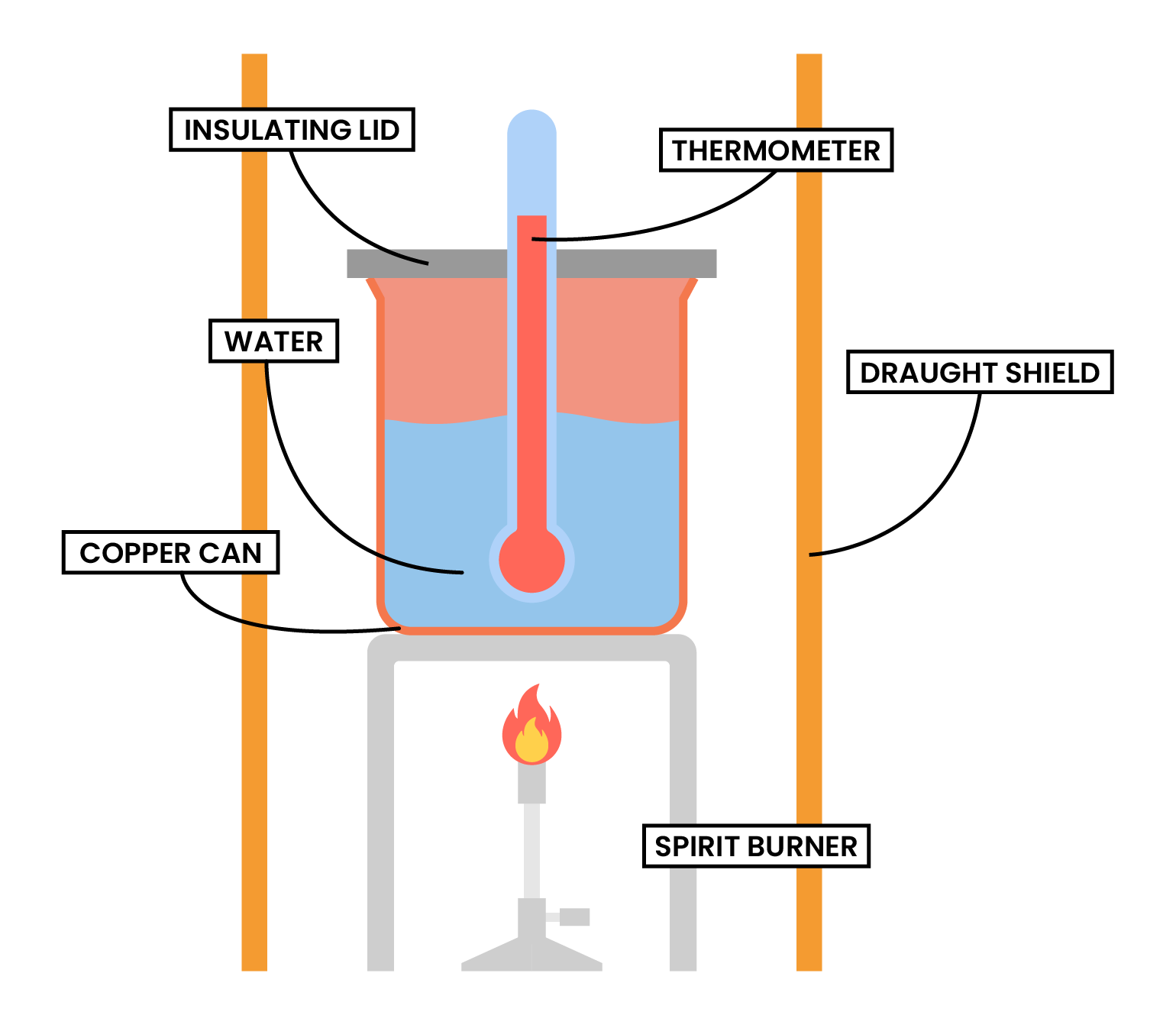

© 2025 Studia Academy. All rights reserved.