REVISION NOTES
4.7.1C Know that esters contain the functional group -COO-
The functional group of esters is
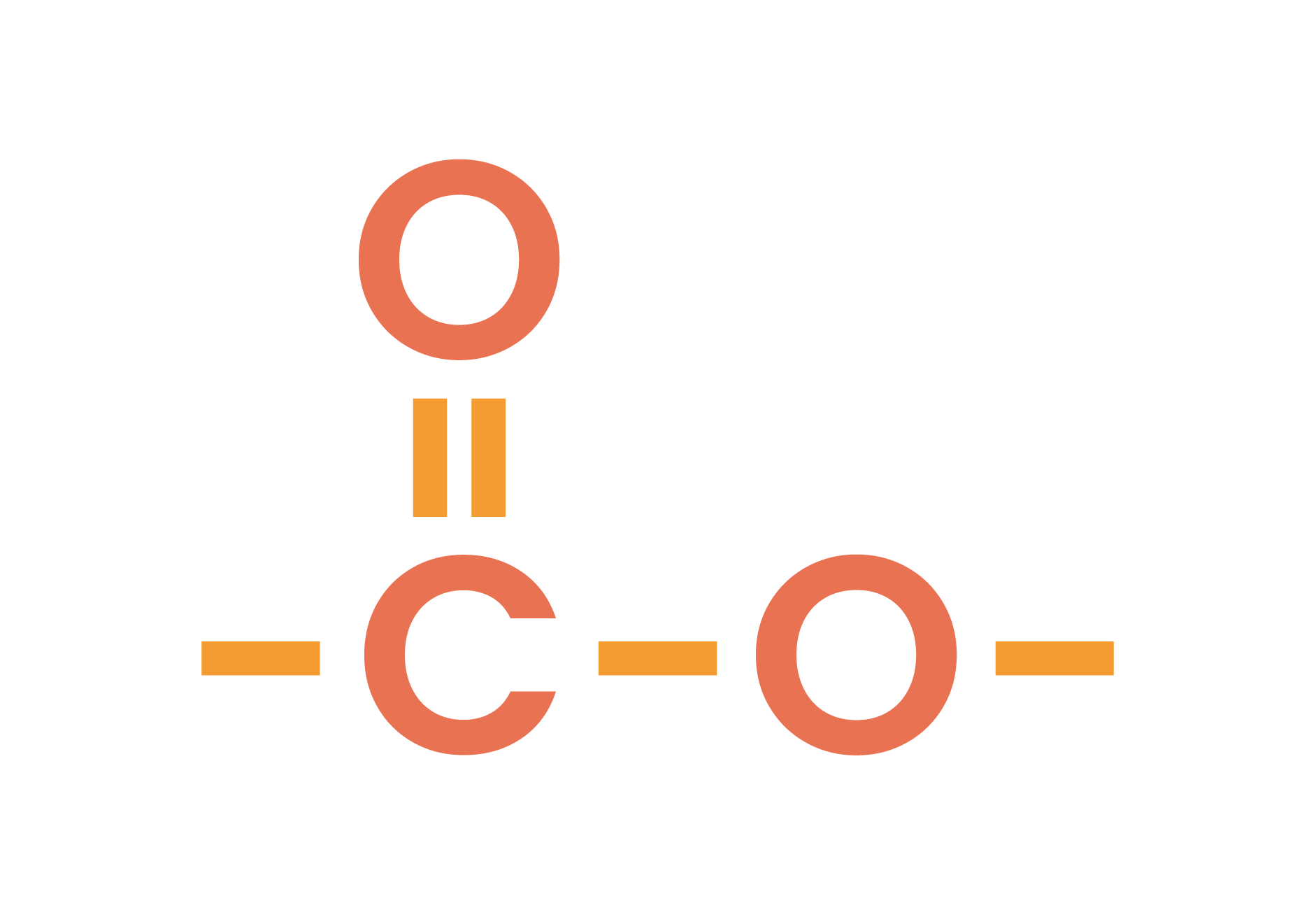
4.7.2C Know that ethyl ethanoate is the ester produced when ethanol and ethanoic acid react in the presence of an acid catalyst
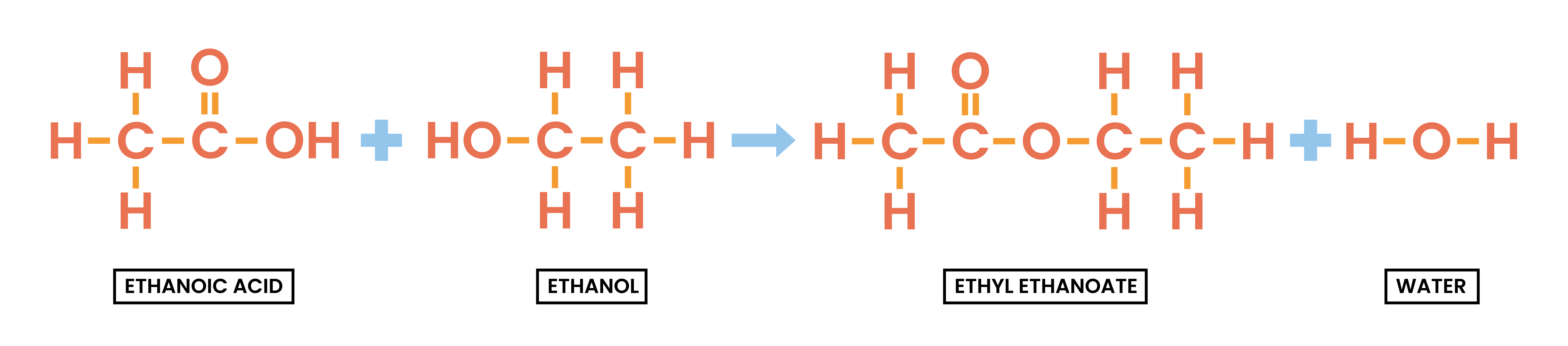
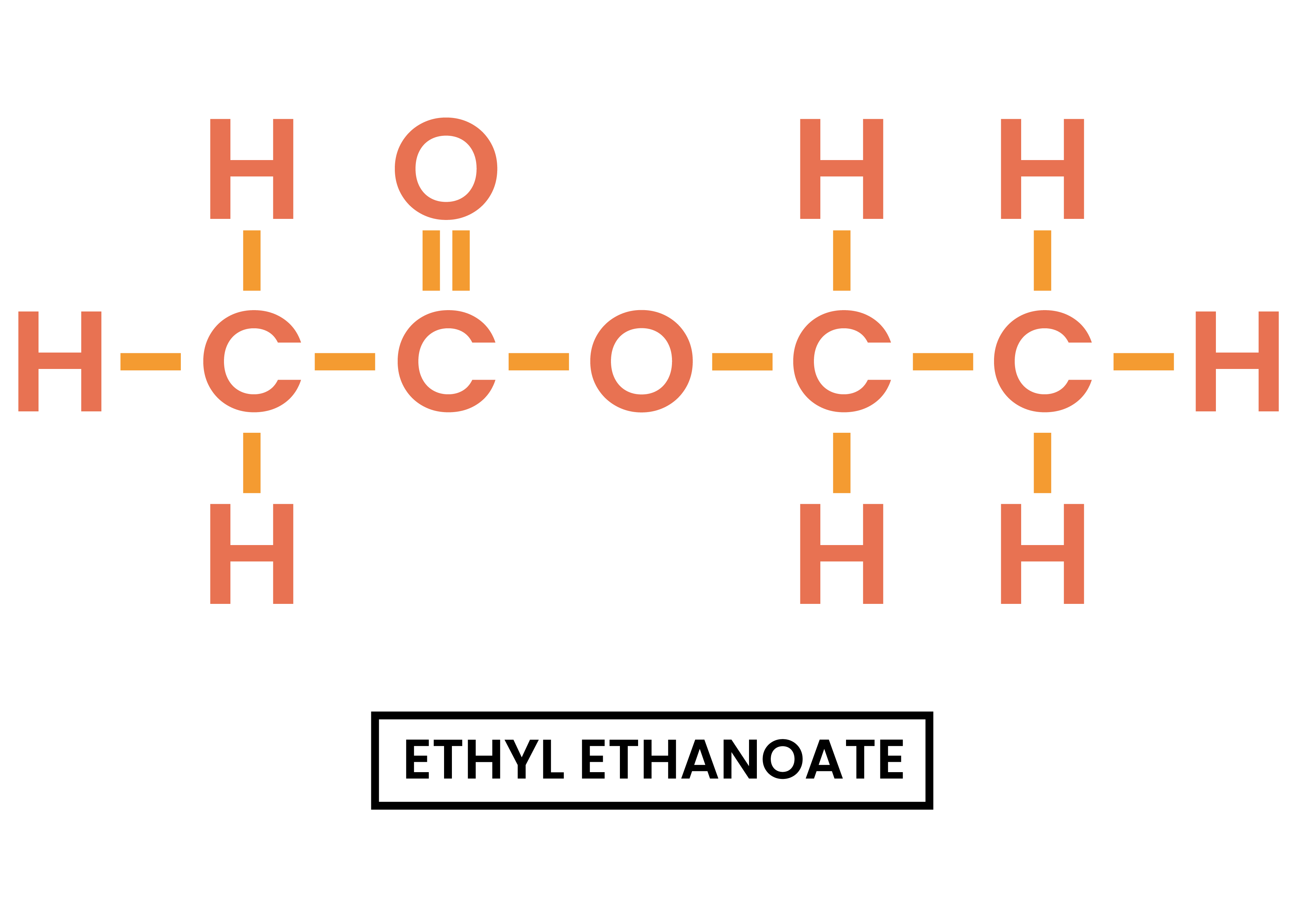
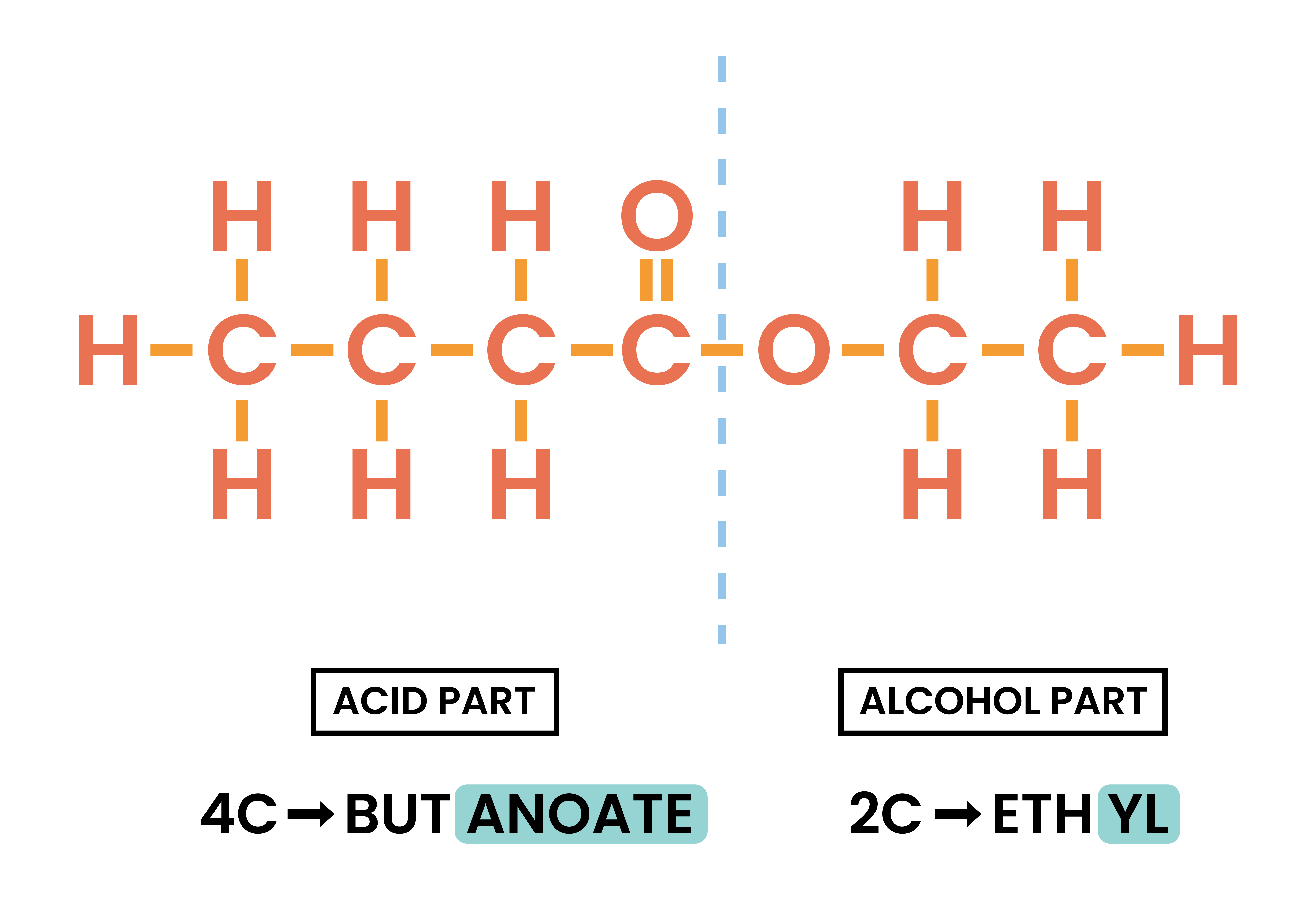
4.7.3C Understand how to write the structural and displayed formulae of ethyl ethanoate
ETHYL ETHANOATE
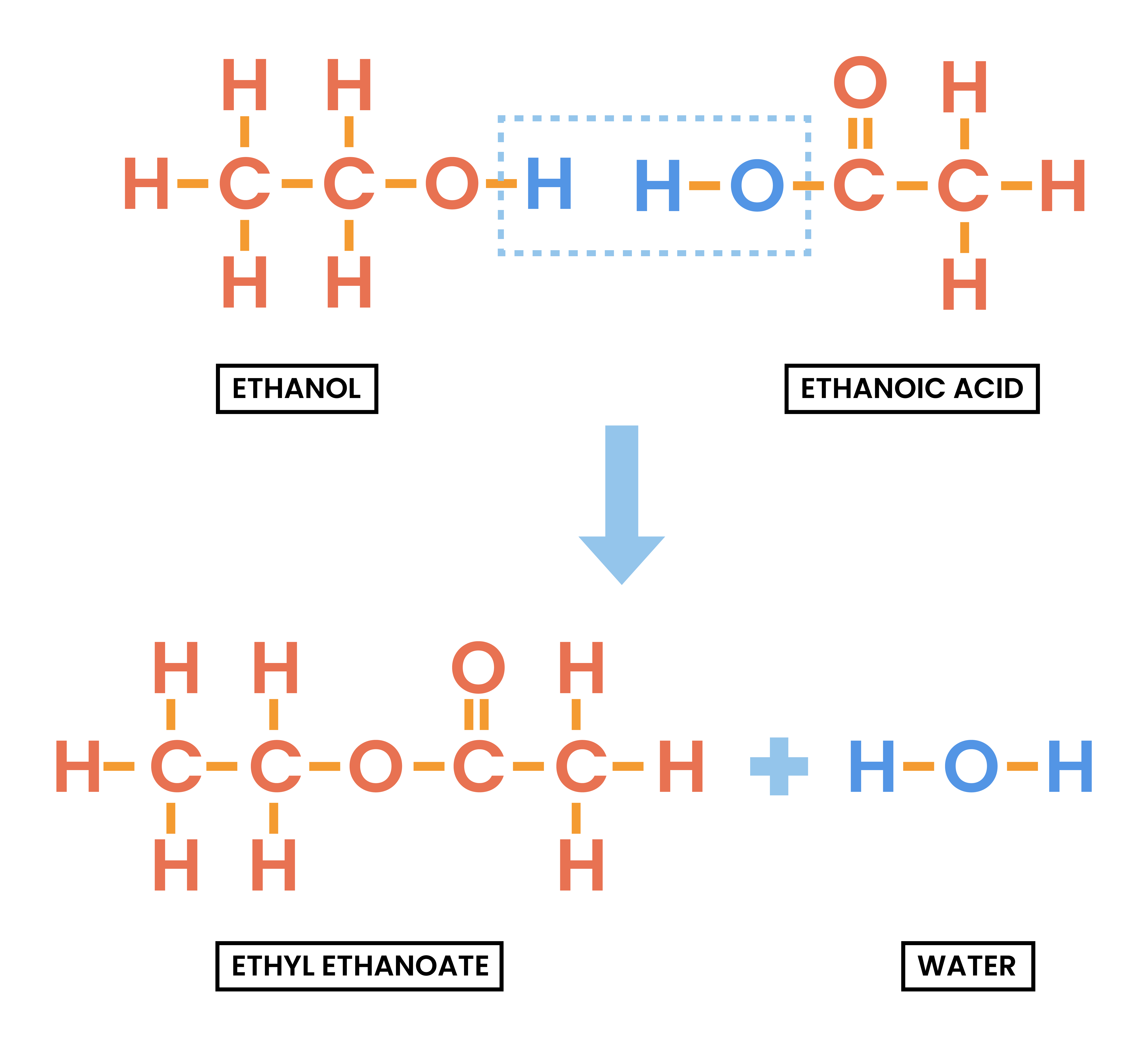
4.7.4C Understand how to write the structural and displayed formulae of an ester, given the name or formula of the alcohol and carboxylic acid from which it is formed and vice versa
Esterification
4.7.5C Know that esters are volatile compounds with distinctive smells and are used as food flavourings and in perfumes
USES OF ESTERS
4.7.6C Practical: prepare a sample of an ester such as ethyl ethanoate
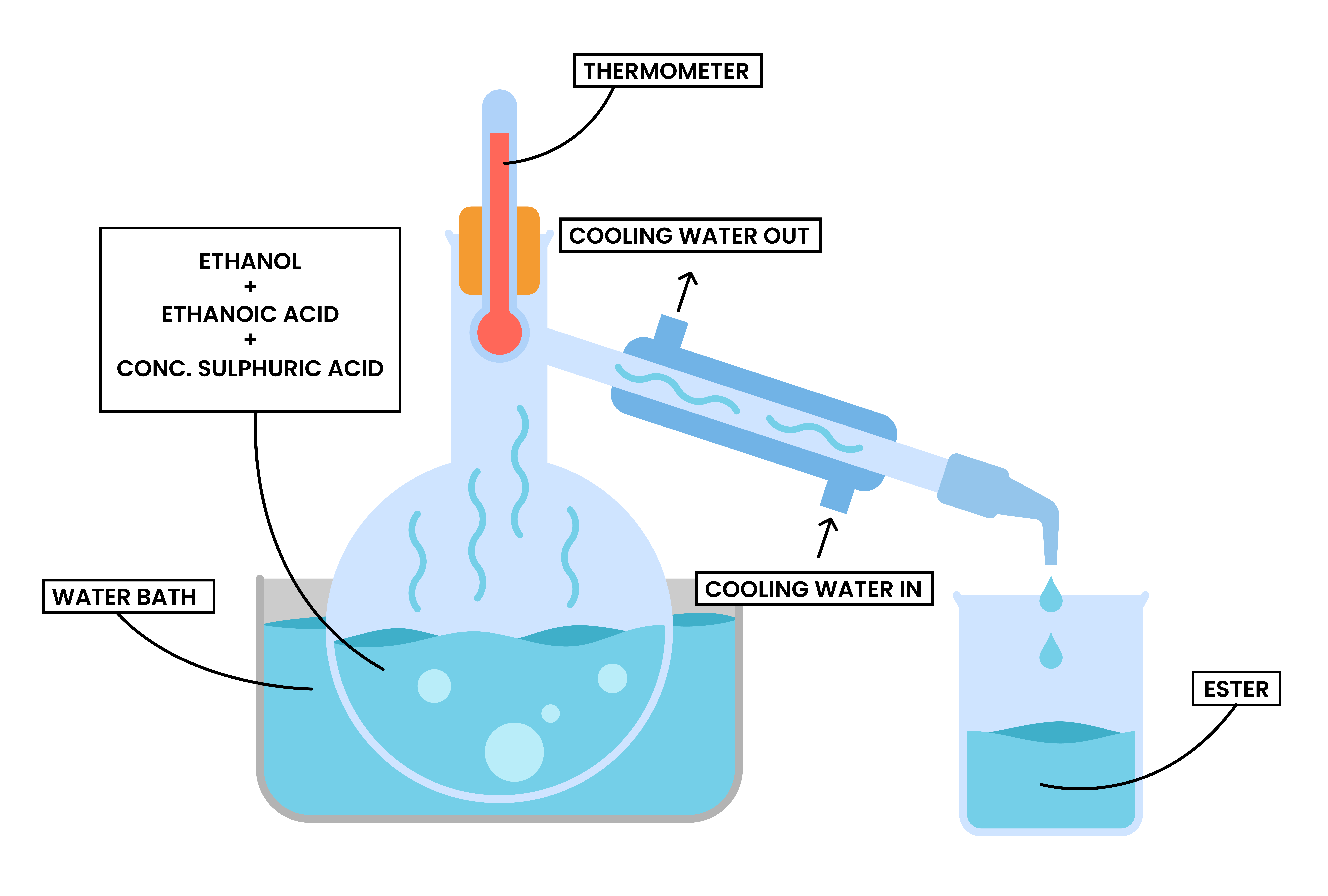
METHODS

© 2025 Studia Academy. All rights reserved.