REVISION NOTES
4.2.1 Know that crude oil is a mixture of hydrocarbons
CRUDE OIL
4.2.2 Describe how the industrial process of fractional distillation separates crude oil into fractions
FRACTIONAL DISTILLATION
PROCESS IN SEPARATING CRUE OILS TO FRACTIONS
4.2.3 Know the names and uses of the main fractions obtained from crude oil: refinery gases, gasoline, kerosene, diesel, fuel oil and bitumen
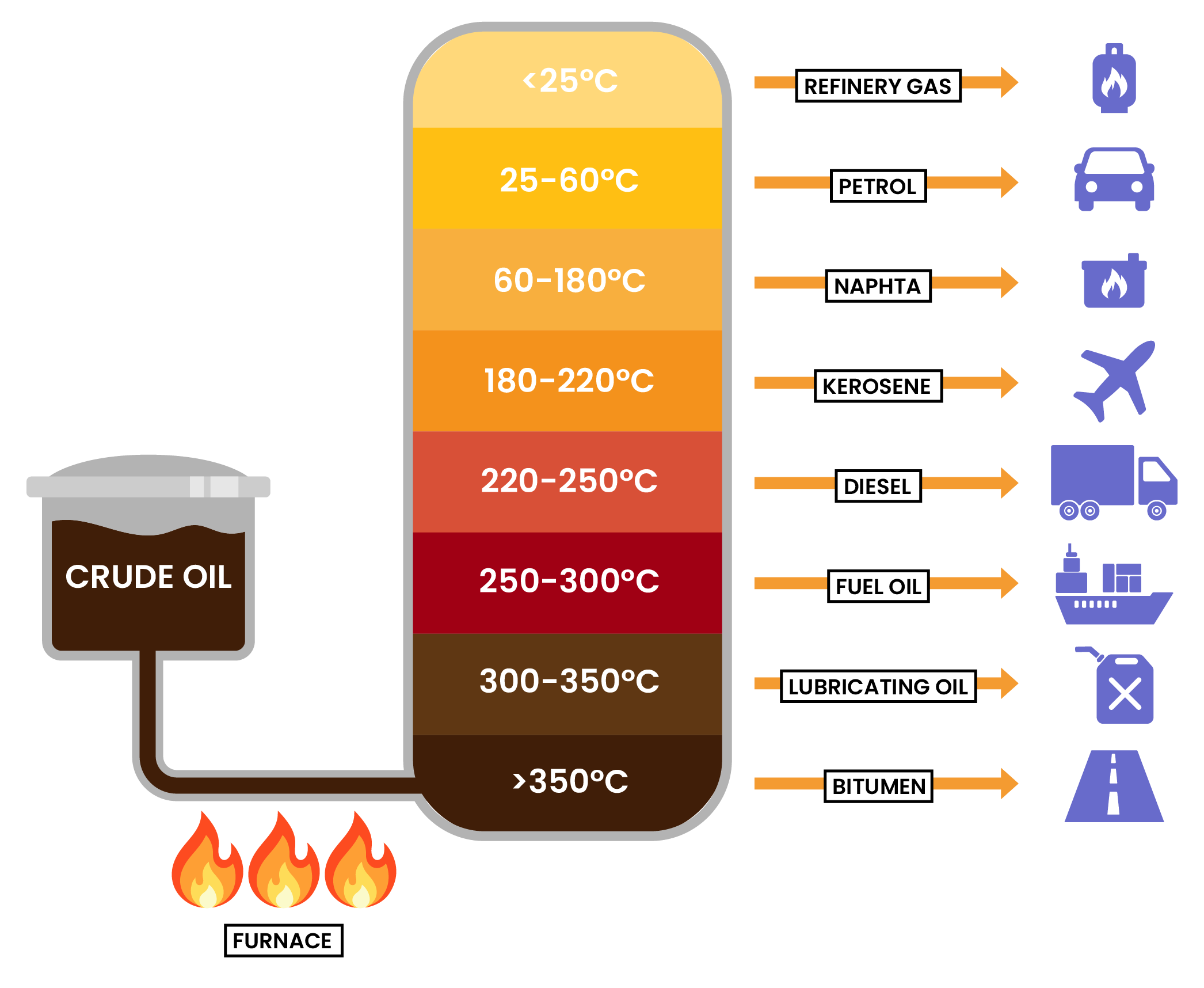
4.2.4 Know the trend in colour, boiling point and viscosity of the main fractions
4.2.5 Know that a fuel is a substance that, when burned, releases heat energy
FUEL
A substance that releases energy as heat when burned
4.2.6 Know the possible products of complete and incomplete combustion of hydrocarbons with oxygen in the air
COMPLETE COMBUSTION OF HYDROCARBONS
hydrocarbon + oxygen [sufficient] → carbon dioxide + water
INCOMPLETE COMBUSTION OF HYDROCARBONS
hydrocarbon + oxygen [insufficient] → carbon / carbon monoxide + water
4.2.7 understand why carbon monoxide is poisonous, in terms of its effect on the capacity of blood to transport oxygen
references to haemoglobin are not required
CARBON MONOXIDE
4.2.8 Know that, in car engines, the temperature reached is high enough to allow nitrogen and oxygen from air to react, forming oxides of nitrogen
FORMATION OF OXIDES OF NITROGEN [TITLE]
nitrogen + oxygen → nitrogen monoxide / nitrogen dioxide
N2 + O2 → NO / NO2
4.2.9 Explain how the combustion of some impurities in hydrocarbon fuels results in the formation of sulfur dioxide
FORMATION OF SULPHUR DIOXIDE
sulphur + oxygen → sulphur dioxide
S + O2 → SO2
4.2.10 Understand how sulfur dioxide and oxides of nitrogen oxides contribute to acid rain
When sulphur dioxide and oxides of nitrogen are emitted into the atmosphere, they react with water (rainwater) to form acids
2SO2 + 2H2O + O2 → 2H2SO4
4NO2 + 2H2O + O2 → 4HNO3
4.2.11 Describe how long-chain alkanes are converted to alkenes and shorter-chain alkanes by catalytic cracking (using silica or alumina as the catalyst and a temperature in the range of 600–700 C)
LONG-CHAIN ALKANES
CATALYTIC CRACKING
4.2.12 explain why cracking is necessary, in terms of the balance between supply and demand for different fractions

© 2025 Studia Academy. All rights reserved.