REVISION NOTES
4.1.1 Know that a hydrocarbon is a compound of hydrogen and carbon only
Hydrocarbon is a compound containing hydrogen and carbon only.
4.1.2 Understand how to represent organic molecules using empirical formulae, molecular formulae, general formulae, structural formulae and displayed formulae
4.1.3 Know what is meant by the terms homologous series, functional group and isomerism
HOMOLOGOUS SERIES
FUNCTIONAL GROUP
ISOMERISM
More will be discussed throughout this topic.
4.1.4 Understand how to name compounds relevant to this specification using the rules of international Union of Pure and Applied Chemistry (IUPAC) nomenclature students will be expected to name compounds containing up to six carbon atoms
PREFIXES (BEGINNING OF THE NAME)
SUFFIXES
4.1.5 Understand how to write the possible structural and displayed formulae of an organic molecule given its molecular formula
If the molecular formula given is C2H6:
Be aware of isomers
Example: C5H12
To name the alkane isomers:
Consider the number of carbons in the longest continuous carbon chain.
Know that the CH3 group is called the “methyl” group .
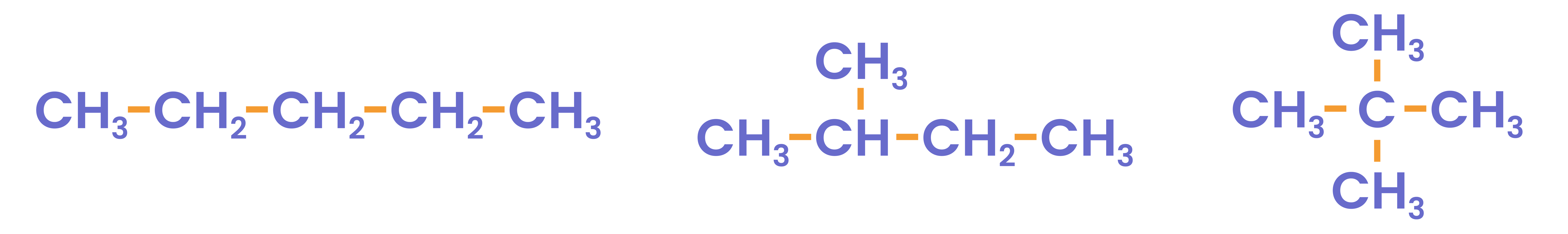
4.1.6 understand how to classify reactions of organic compounds as substitution, addition and combustion
Knowledge of reaction mechanisms is not required
ADDITION REACTIONS
SUBSTITUTION REACTIONS
COMBUSTION REACTIONS

© 2025 Studia Academy. All rights reserved.