REVISION NOTES
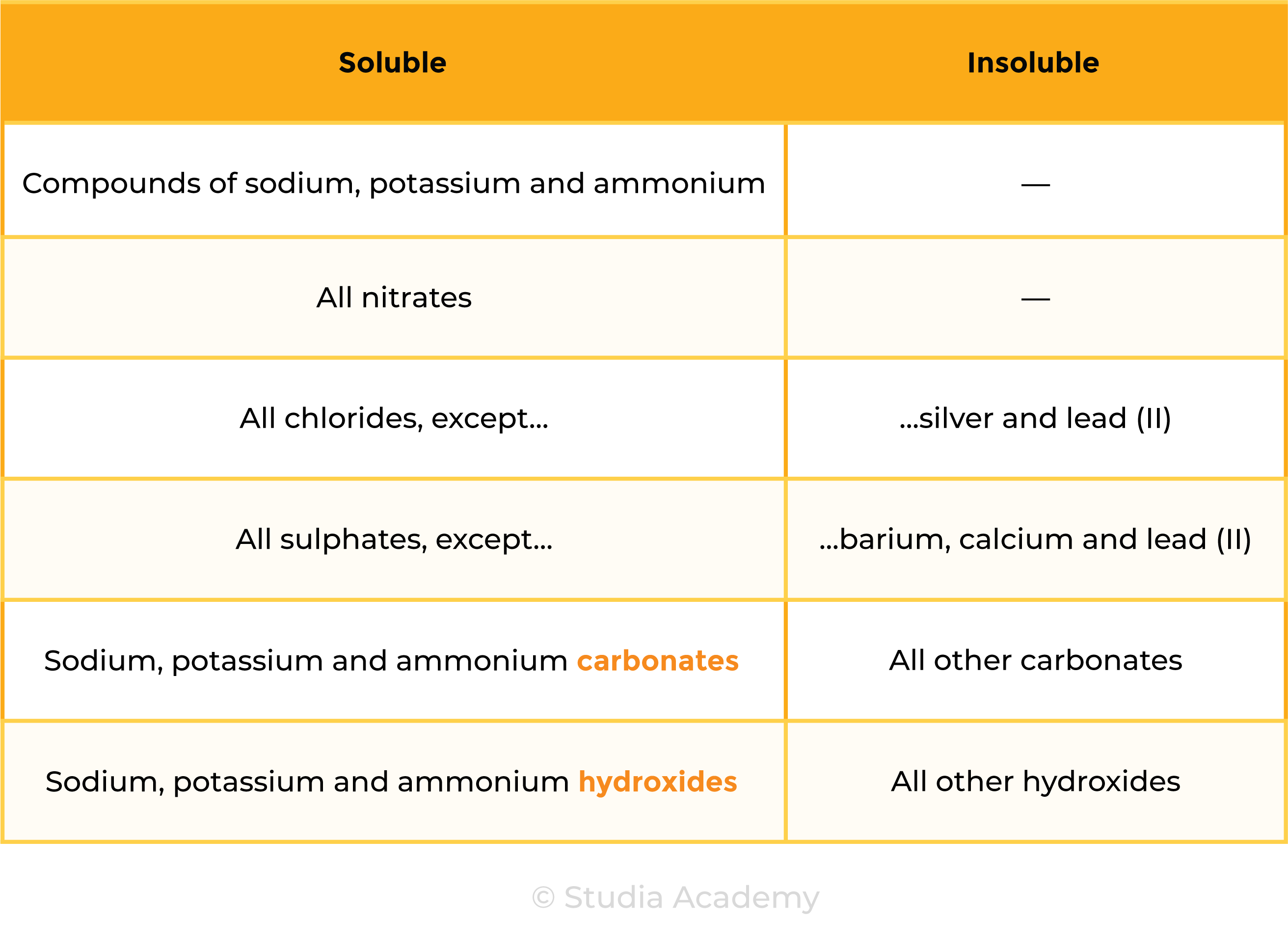
2.7.1 Know the general rules for predicting the solubility of ionic compounds in water:
SOLUBILITY OF SALTS
2.7.2 Understand acids and bases in terms of proton transfer
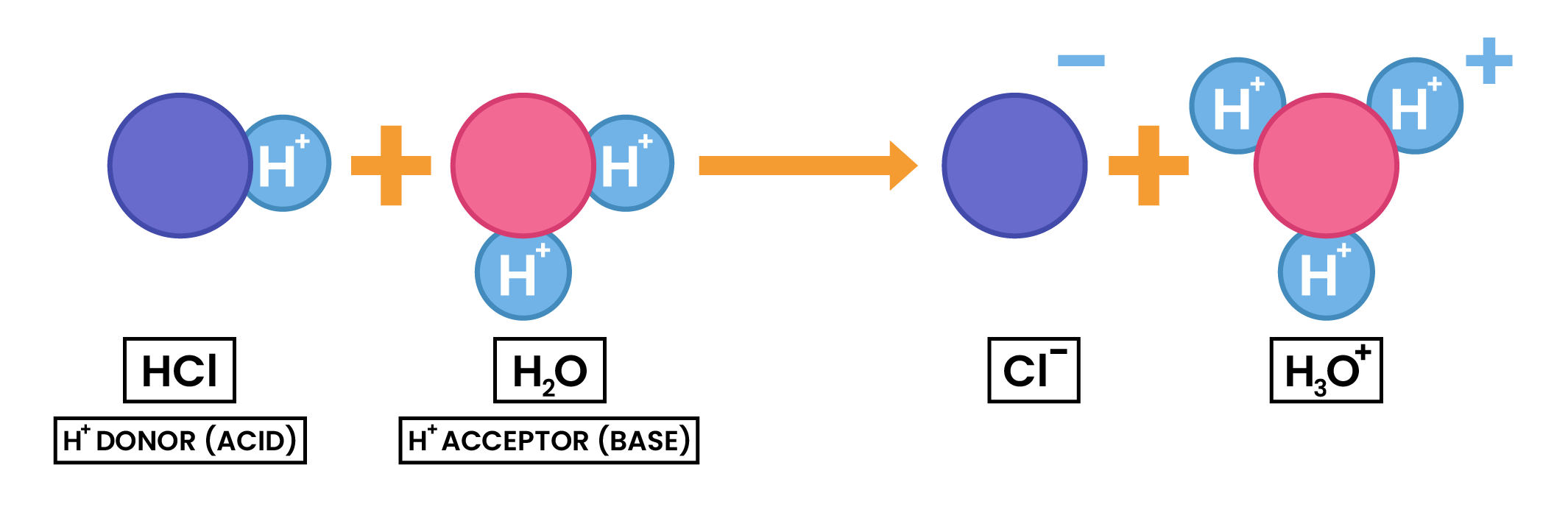
2.7.3 Understand that an acid is a proton donor and a base is a proton acceptor
Refer to 2.7.2
2.7.4 Describe the reactions of hydrochloric acid, sulfuric acid and nitric acid with metals, bases and metal carbonates (excluding the reactions between nitric acid and metals) to form salts
Reaction 1 Acid + Metal
Acid + Metal → Salt + Hydrogen
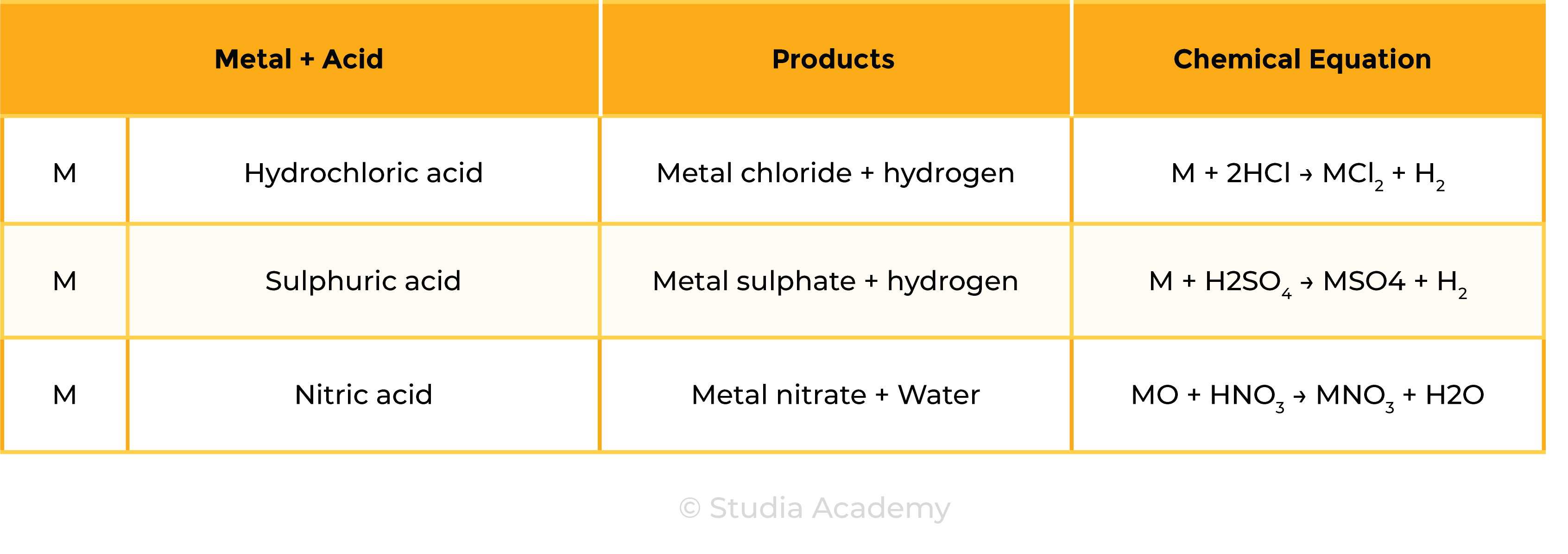
Examples
Examples
Reaction 3 Acid + Metal Carbonate
Acid + Metal carbonate → Salt + Carbon dioxide + Water
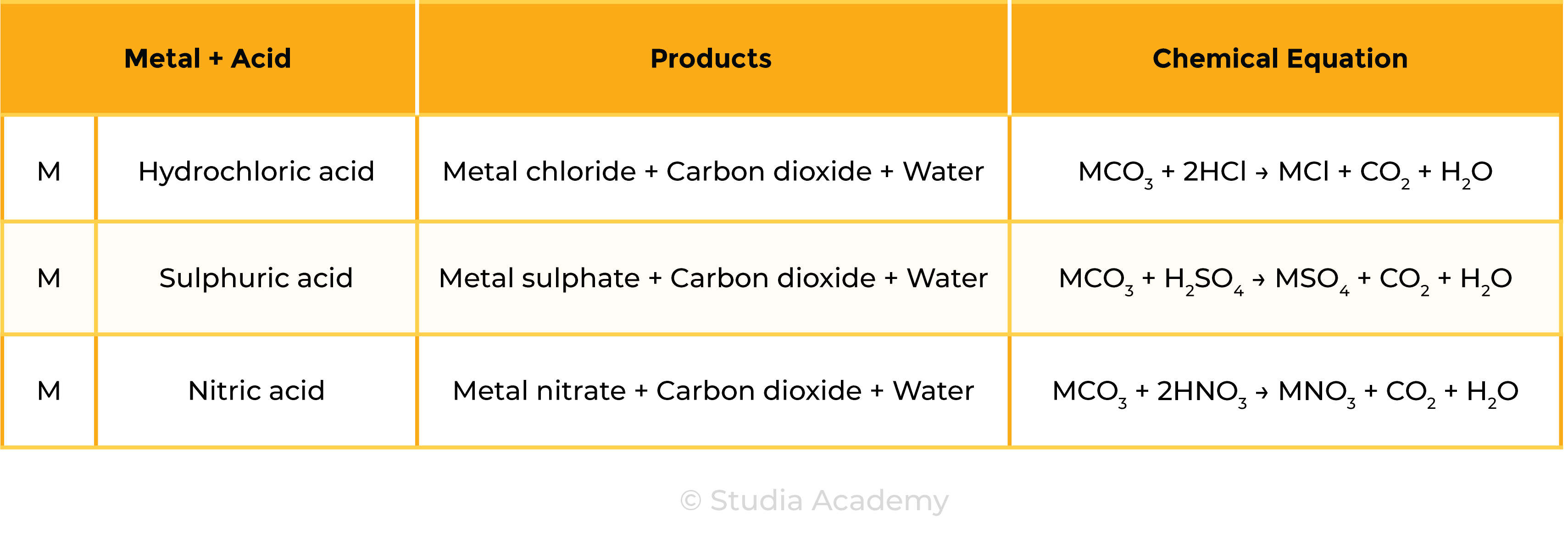
Examples
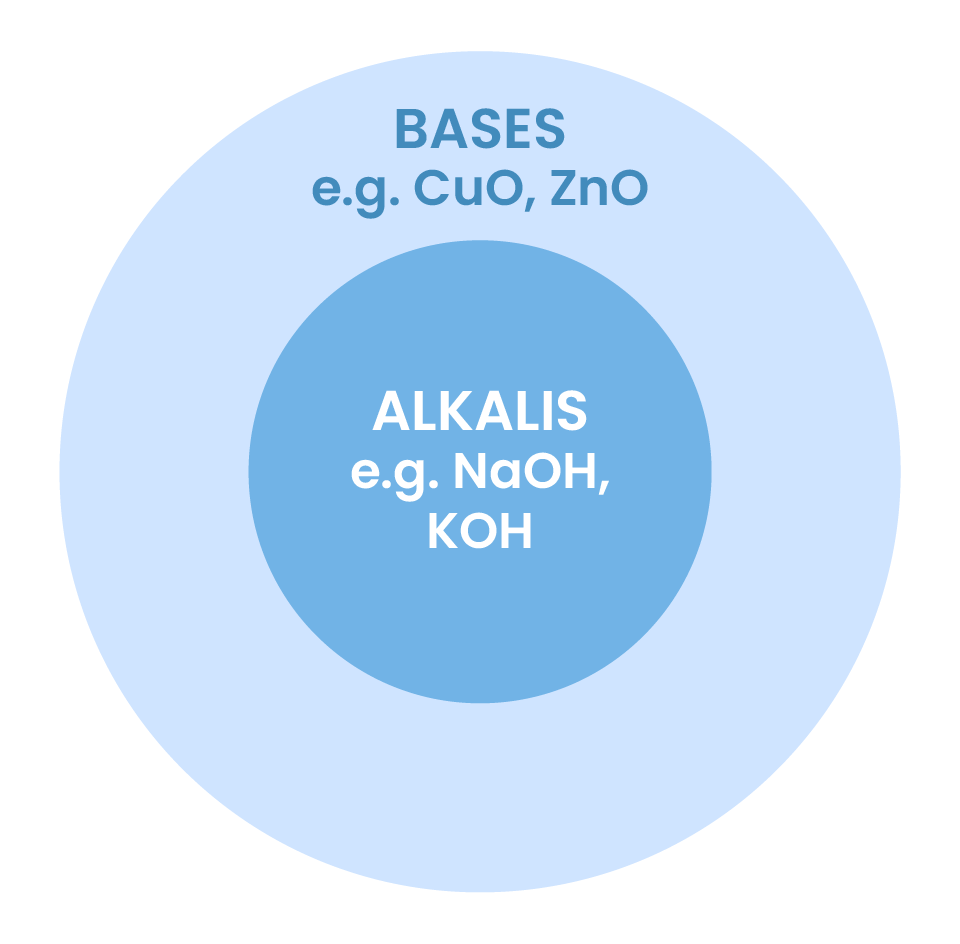
2.7.5 Know that metal oxides, metal hydroxides and ammonia can act as bases, and that alkalis are bases that are soluble in water
Common bases
Alkalis
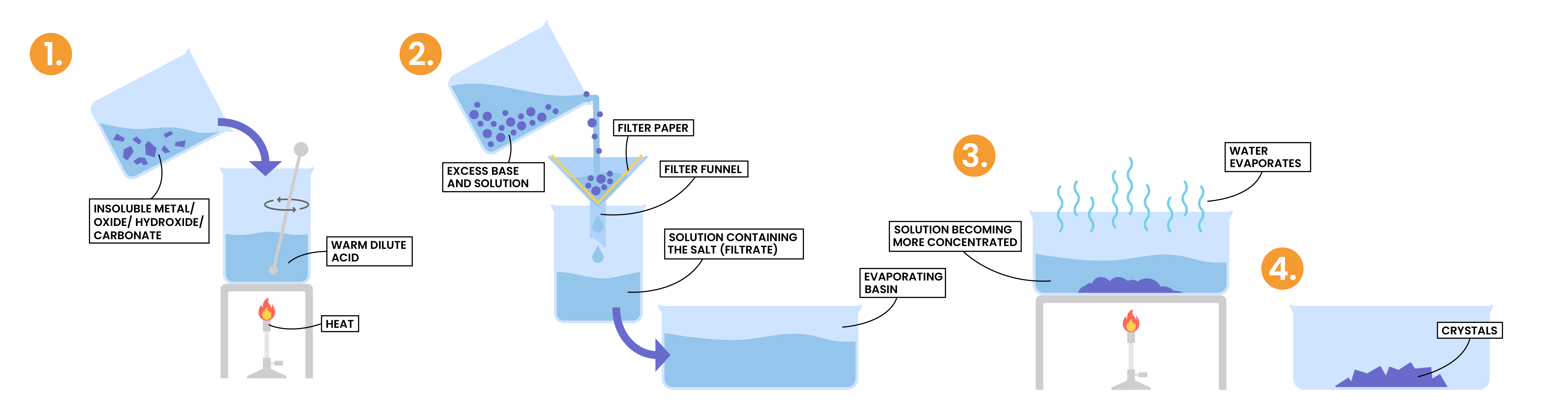
2.7.6 Describe an experiment to prepare a pure, dry sample of a soluble salt, starting from an insoluble reactant
PREPARATION OF SALT
Key points
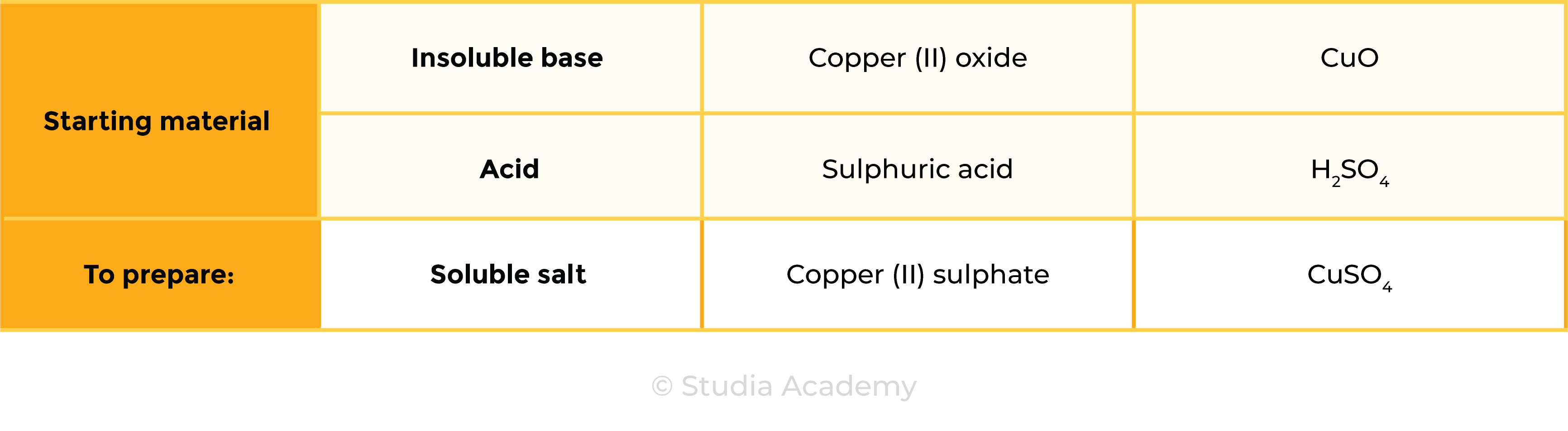
Example: preparation of copper (II) sulphate
CuO (s) + H2SO4 (aq) ⟶ CuSO4 (s) + H2O (l)
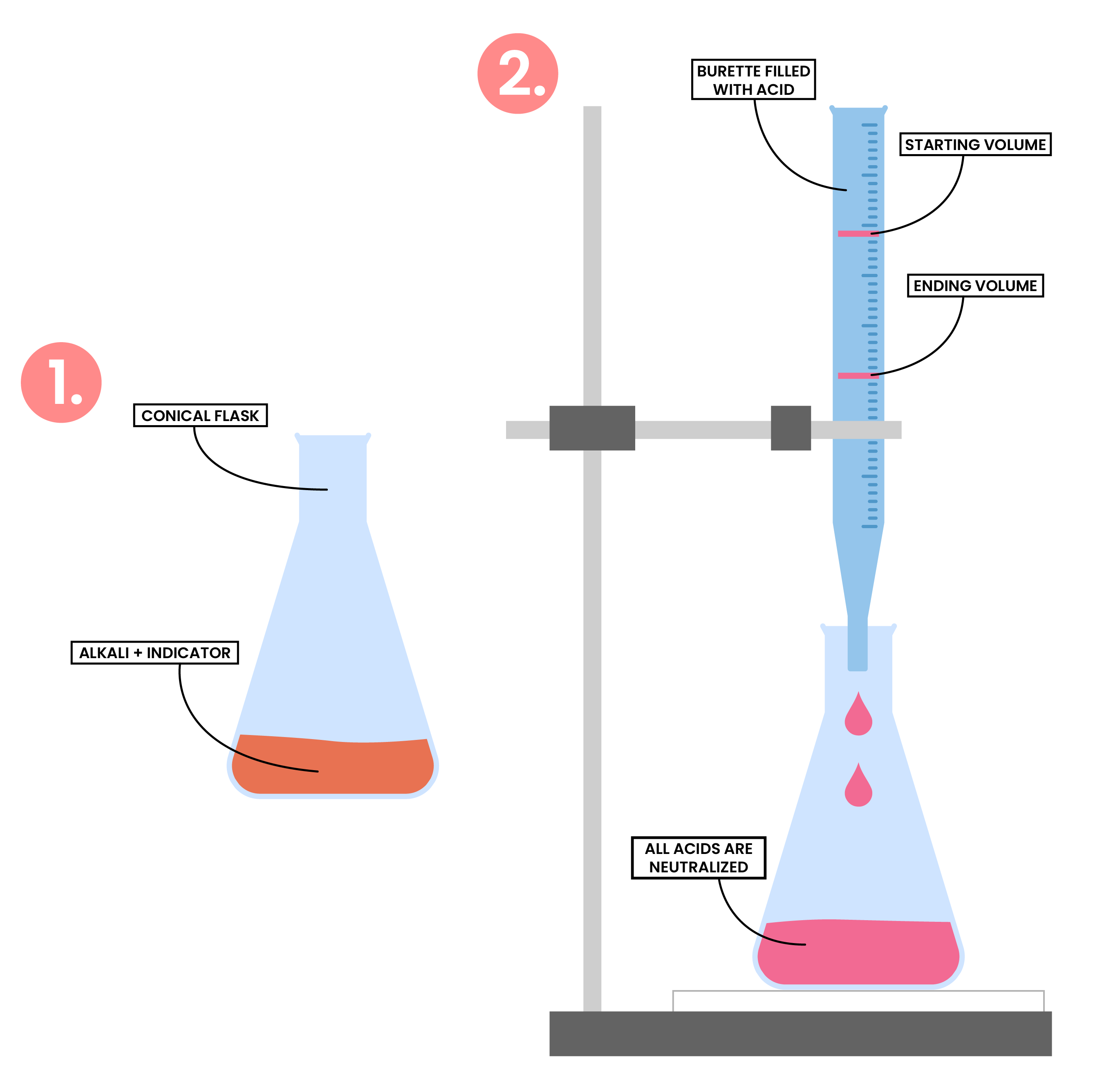
2.7.7C Describe an experiment to prepare a pure, dry sample of a soluble salt, starting from an acid and alkali
PREPARATION OF SOLUBLE SALT METHOD 2
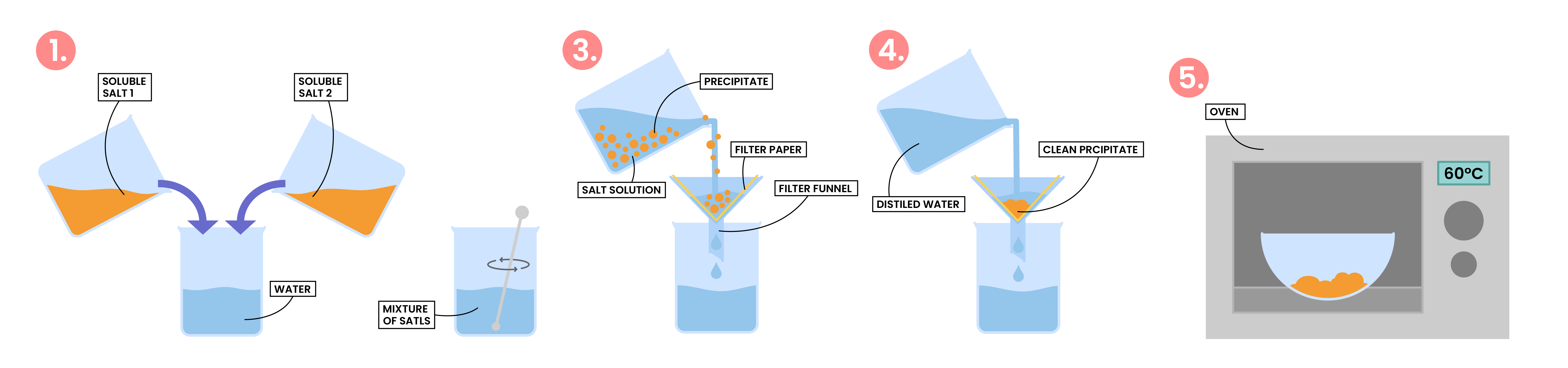
2.7.8C Describe an experiment to prepare a pure, dry sample of an insoluble salt, starting from two soluble reactants
PREPARATION OF INSOLUBLE SALT
Soluble Salt 1 + Soluble Salt 2 ⟶ Insoluble Salt + Soluble Salt 3
AB + CD ⟶ AD + CB
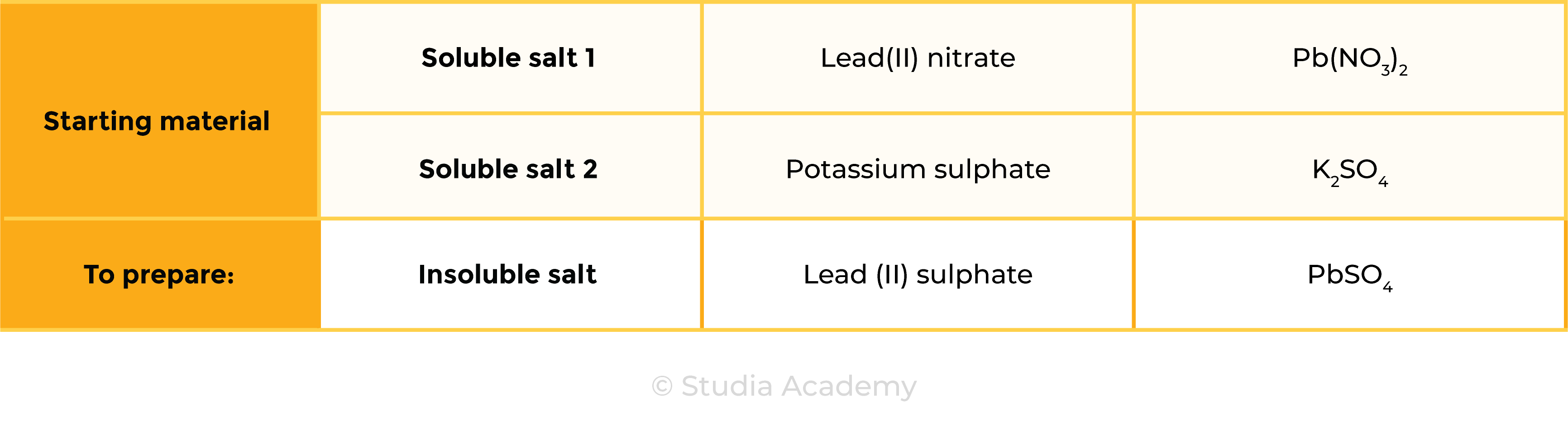
Example: preparation of silver and lead (II) salts
2.7.9 Practical: prepare a sample of pure, dry hydrated copper(II) sulfate crystals starting from copper(II) oxide
copper (II) oxide + sulphuric acid → copper (II) sulphate + water
CuO (s) + H2SO4 (aq) ⟶ CuSO4 (s) + H2O (l)
METHODS
RESULTS
2.7.10C Practical: prepare a sample of pure, dry lead(II) sulfate
lead(II) nitrate + potassium sulphate → lead(II) sulphate + potassium nitrate
Pb(NO3)2 (aq) + K2SO4 (aq) → PbSO4 (s) + 2KNO3 (aq)
METHODS

© 2025 Studia Academy. All rights reserved.