REVISION NOTES
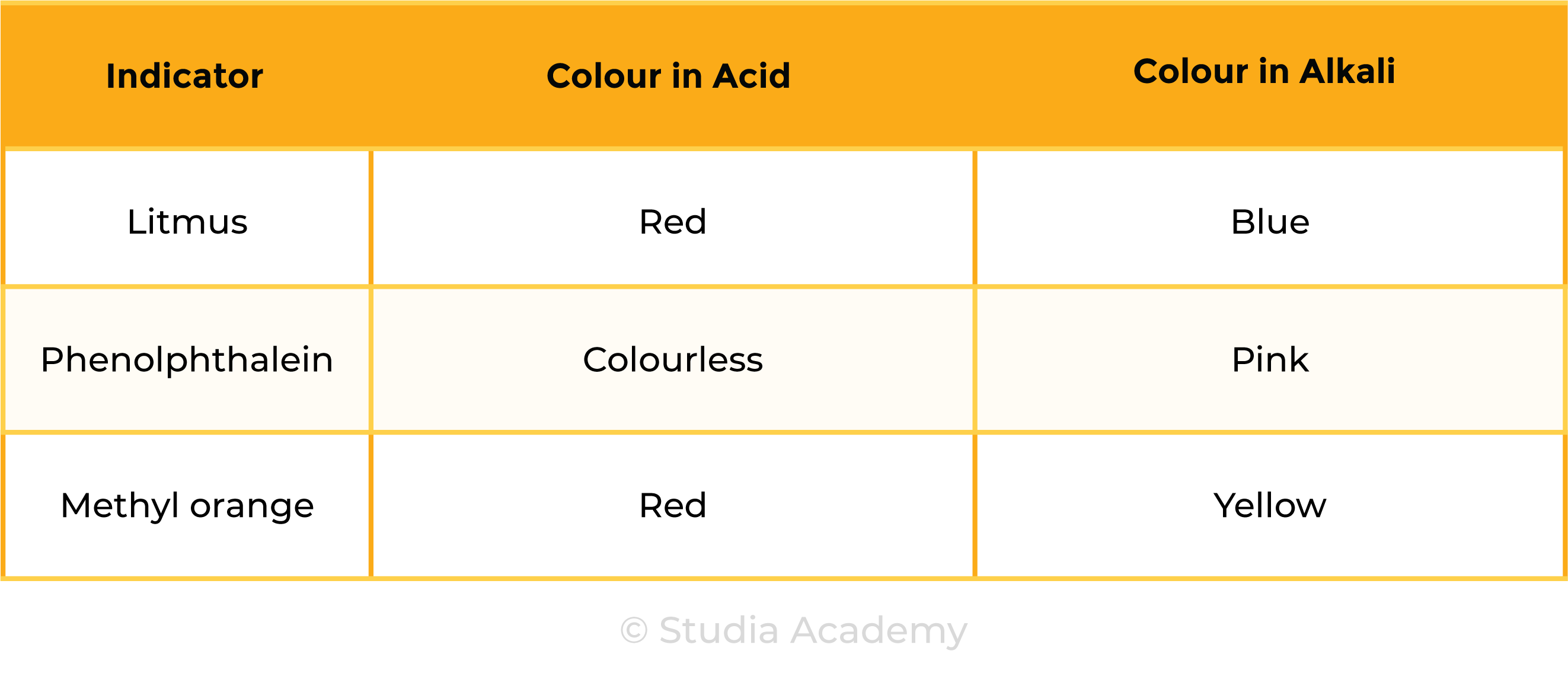
2.6.1 Describe the use of litmus, phenolphthalein and methyl orange to distinguish between acidic and alkaline solutions
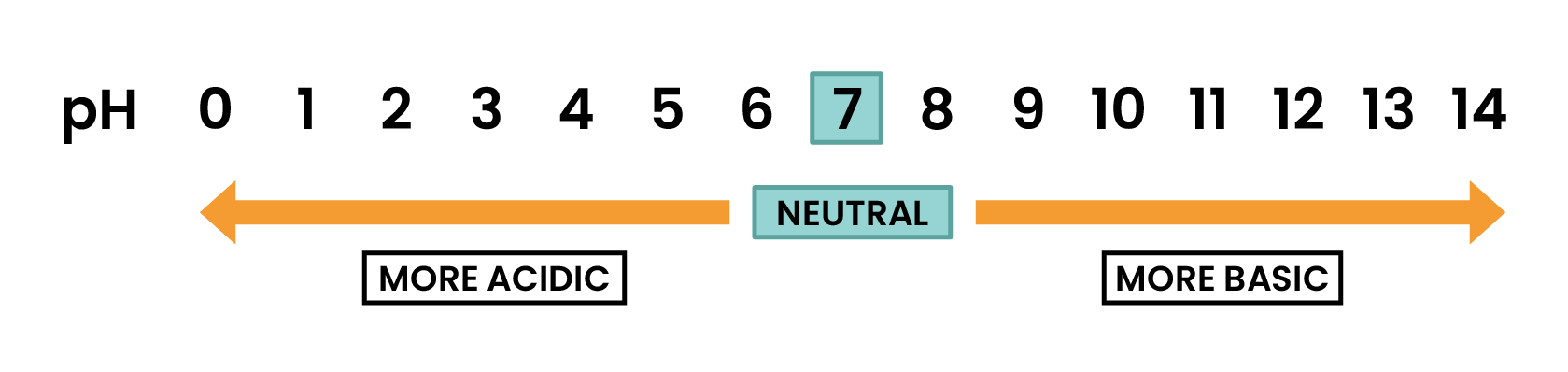
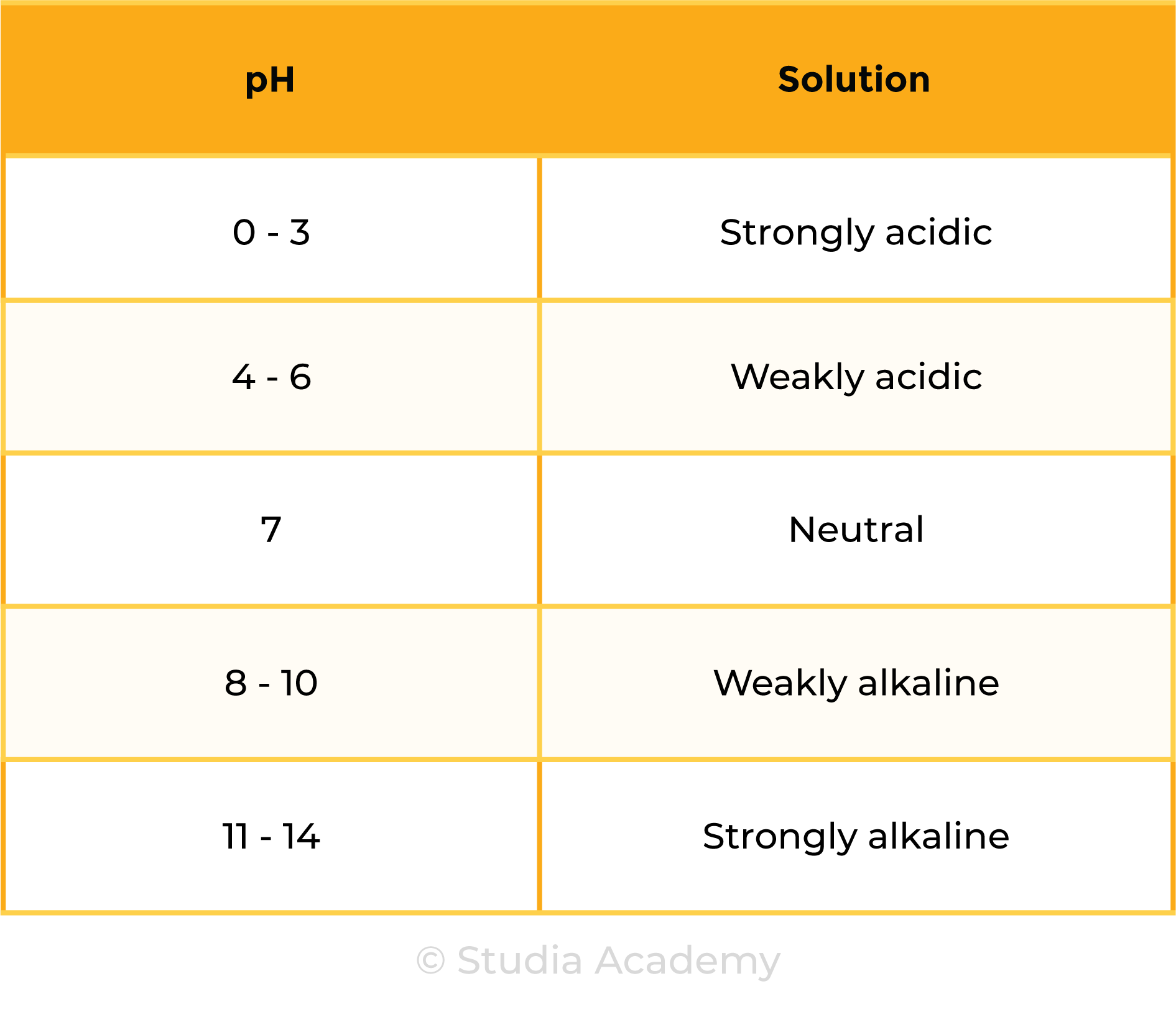
2.6.2 Understand how to use the pH scale, from 0–14, can be used to classify solutions as strongly acidic (0–3), weakly acidic (4–6), neutral (7), weakly alkaline (8–10) and strongly alkaline (11–14)
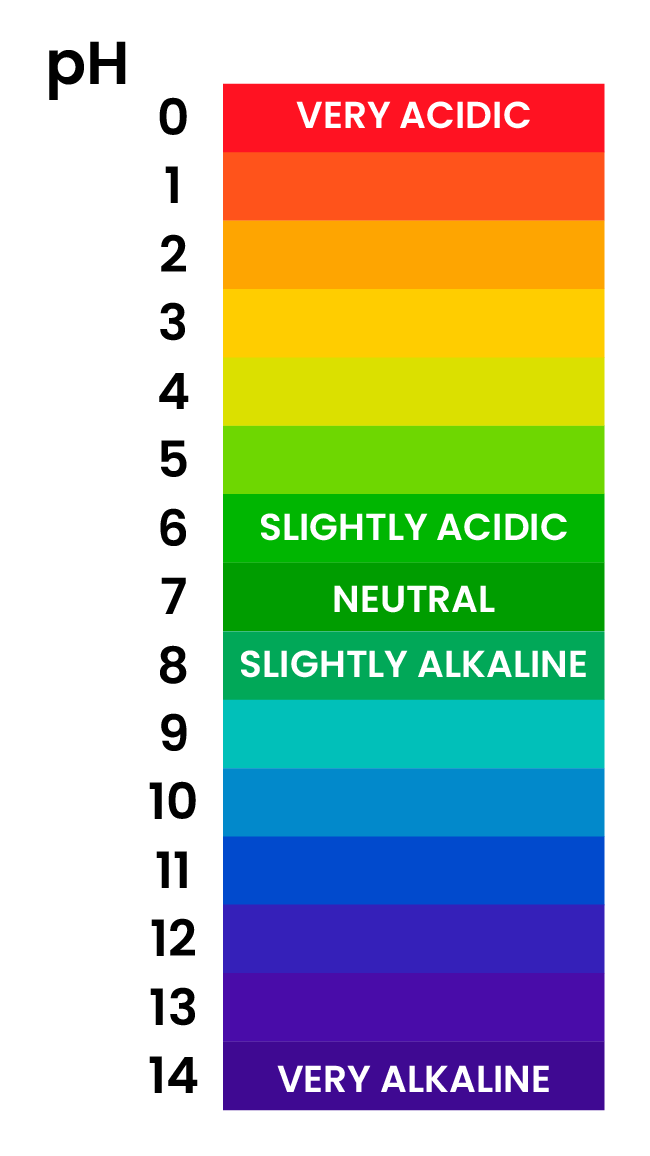
2.6.3 Describe the use of universal indicator to measure the approximate pH value of an aqueous solution
UNIVERSAL INDICATOR
2.6.4 Know that acids in aqueous solution are a source of hydrogen ions and alkalis in a aqueous solution are a source of hydroxide ions
ACIDS
ALKALIS
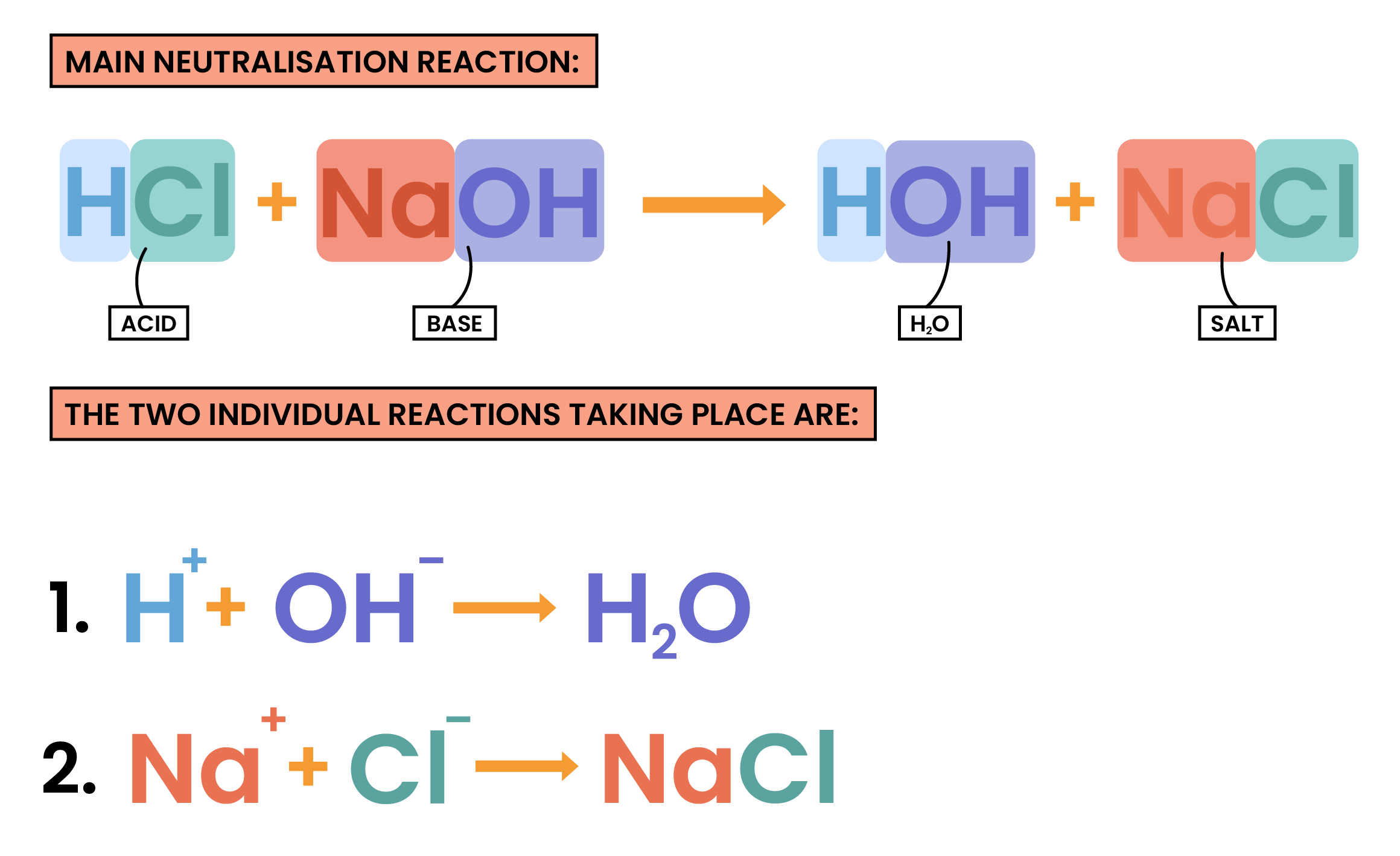
2.6.5 Know that alkalis can neutralise acids
NEUTRALISATION
2.6.6C Describe how to carry out an acid-alkali titration
ACID-ALKALI TITRATION
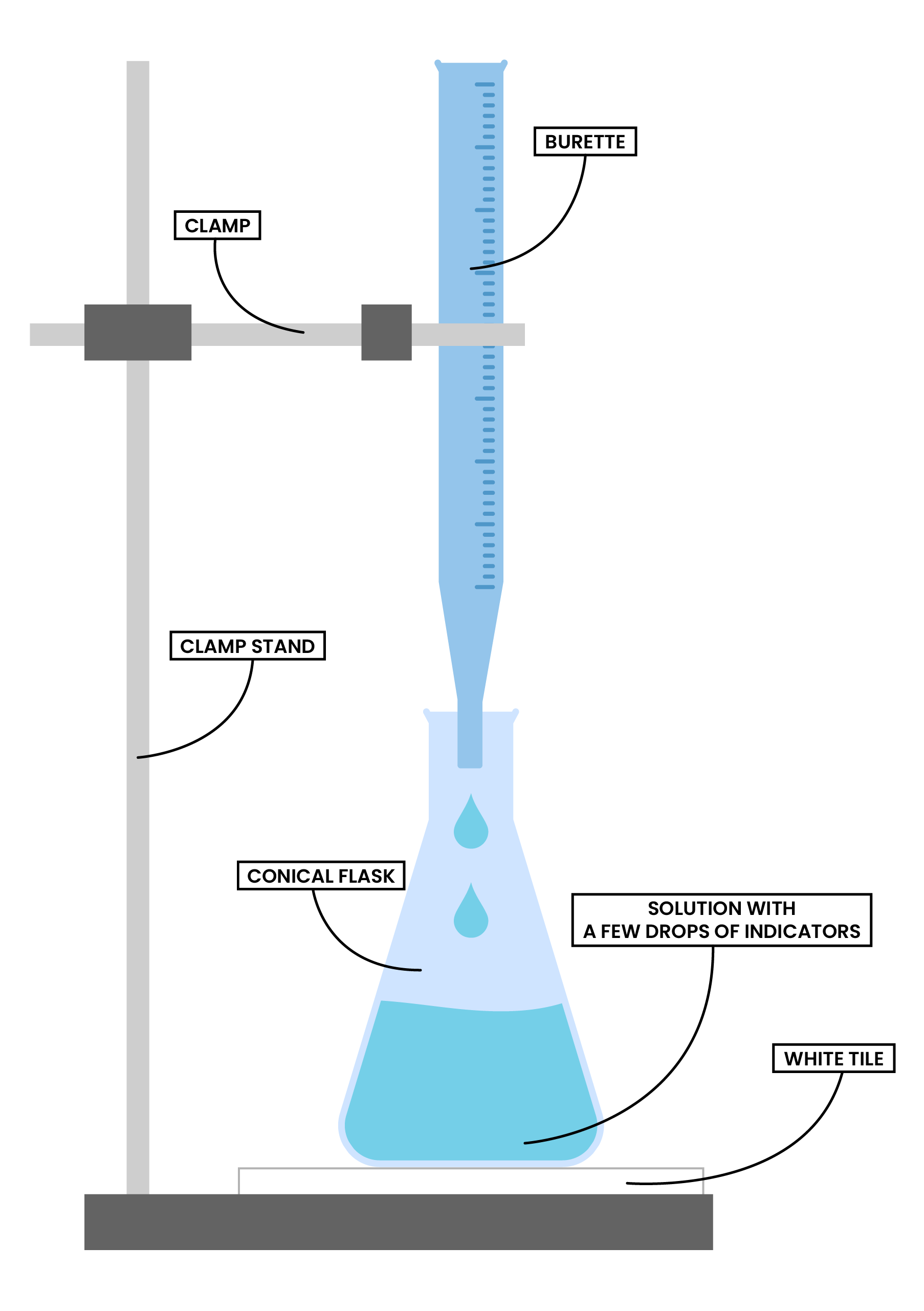
HOW TO CARRY OUT TITRATION

© 2025 Studia Academy. All rights reserved.