REVISION NOTES
2.5.1C Know that most metals are extracted from ores found in the Earth’s crust and that unreactive metals are often found as the uncombined element
2.5.2C Explain how the method of extraction of a metal is related to its position in the reactivity series, illustrated by carbon extraction for iron and electrolysis for aluminium
METHOD OF EXTRACTION

2.5.3C Be able to comment on a metal extraction process, given appropriate information
(detailed knowledge of the processes used in the extraction of a specific metal is not required)
EXTRACTION OF IRON
EXTRACTION OF ALUMINUIM
2.5.4C Explain the uses of aluminium, copper, iron and steel in terms of their properties
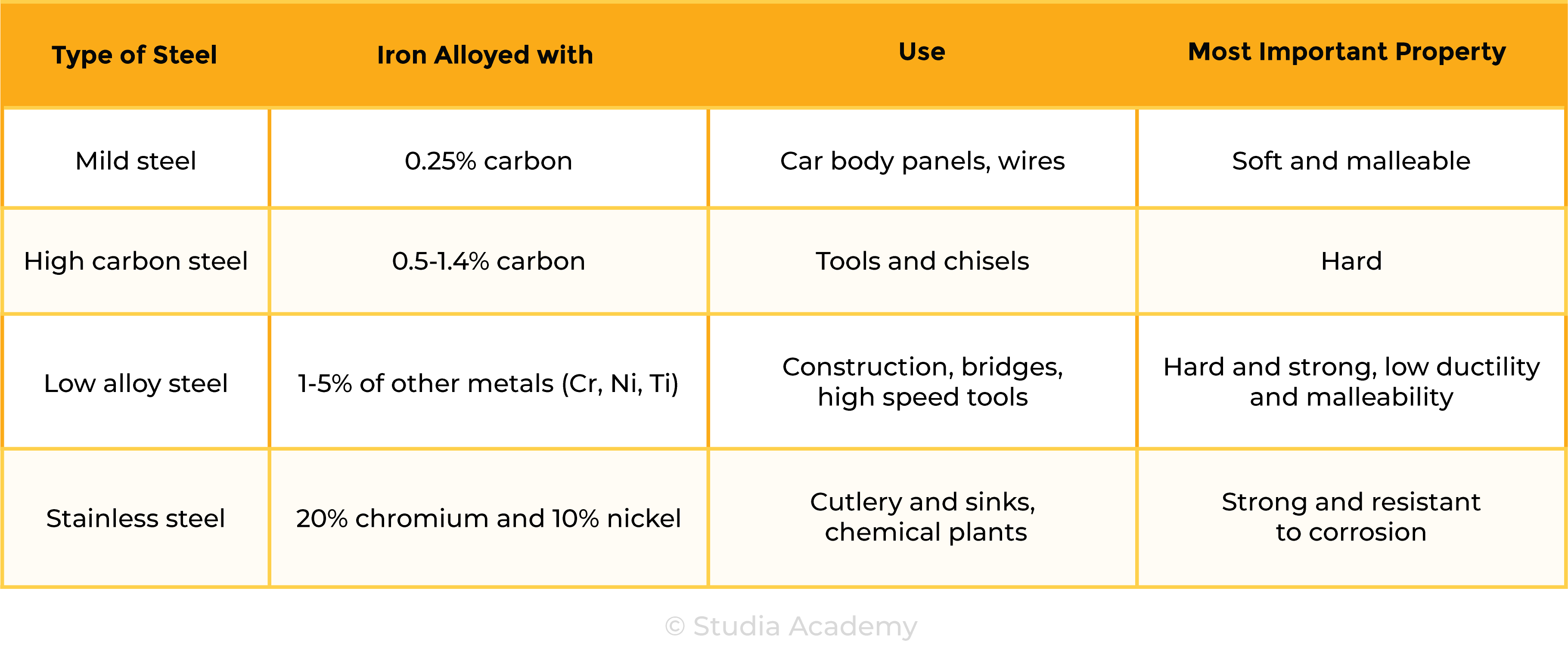
the types of steel will be limited to low-carbon (mild), high-carbon and stainless
Uses of Aluminium
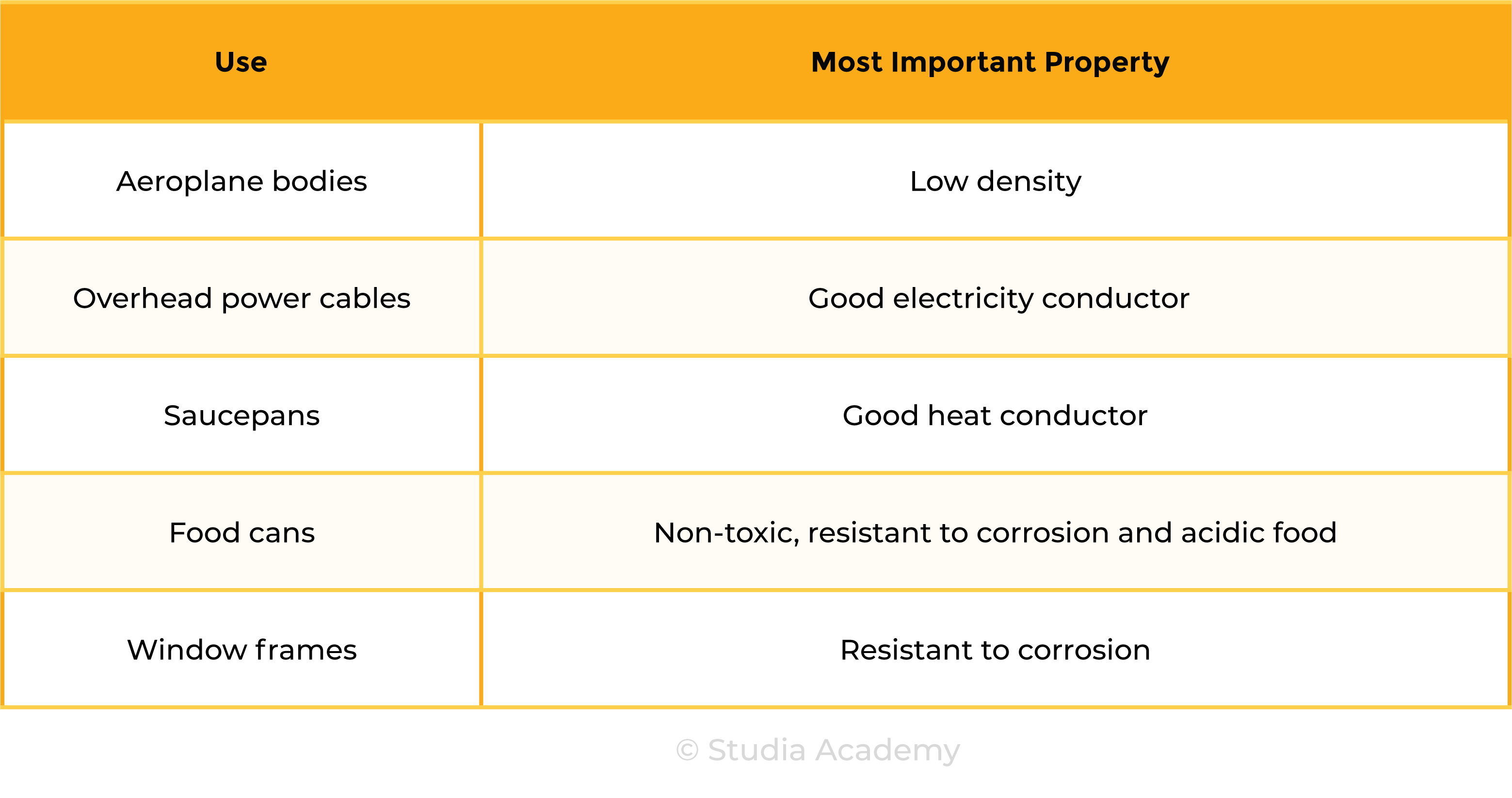
Uses of Copper

Uses of Iron
Uses of Steel
2.5.5C Know that an alloy is a mixture of a metal and one or more elements, usually other metals or carbon
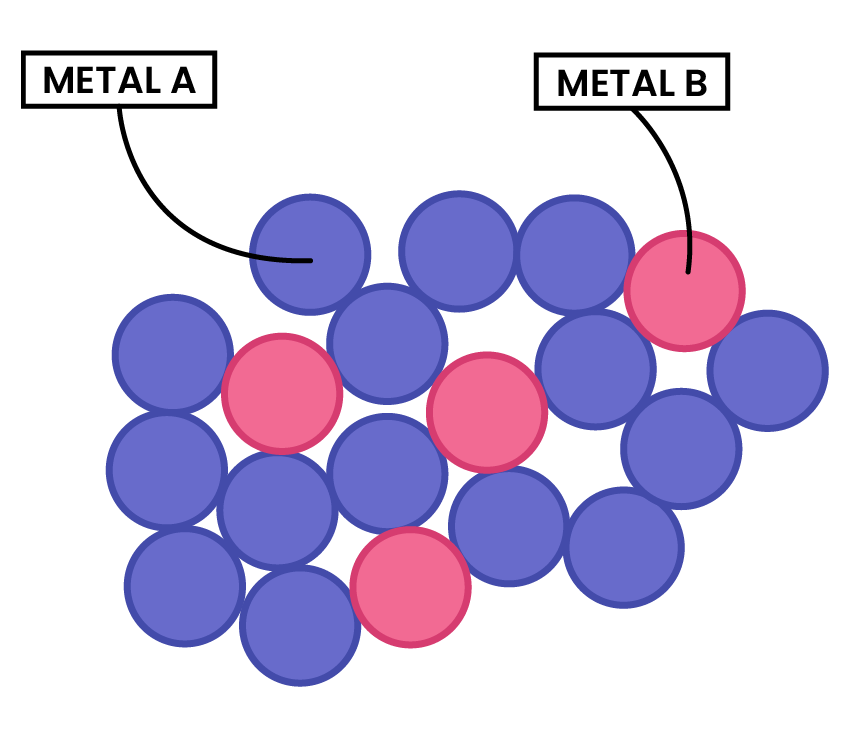
ALLOYS
2.5.6C Explain why alloys are harder than pure metals
PROPERTIES OF ALLOYS

© 2025 Studia Academy. All rights reserved.