REVISION NOTES
2.4.1 Understand how metals can be arranged in a reactivity series based on their reactions with:
REACTIVITY SERIES OF METAL
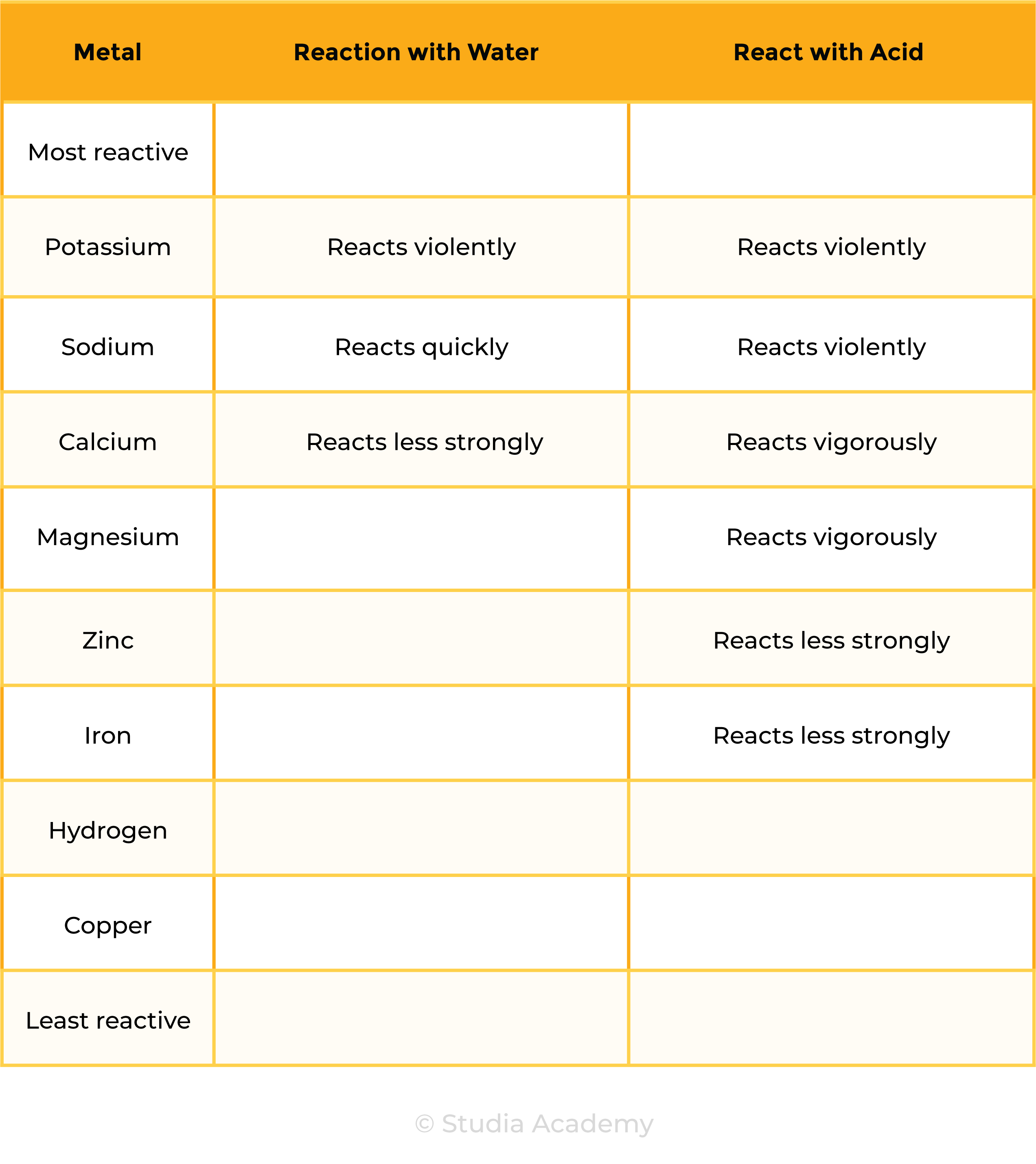
Reaction 1: Metal + Water
Metal + Water → Metal hydroxide + Hydrogen
Ca (s) + 2H2O (l) → Ca(OH)2 (aq) + H2 (g)
Calcium + Water → Calcium hydroxide + Hydrogen
Reaction 2: Metal + Acid
Metal + Acid → Salt + Hydrogen
2K (s) + 2HCl (aq) → 2KCl (s) + H2 (g)
Potassium + Hydrochloric acid → Potassium chloride + Hydrogen
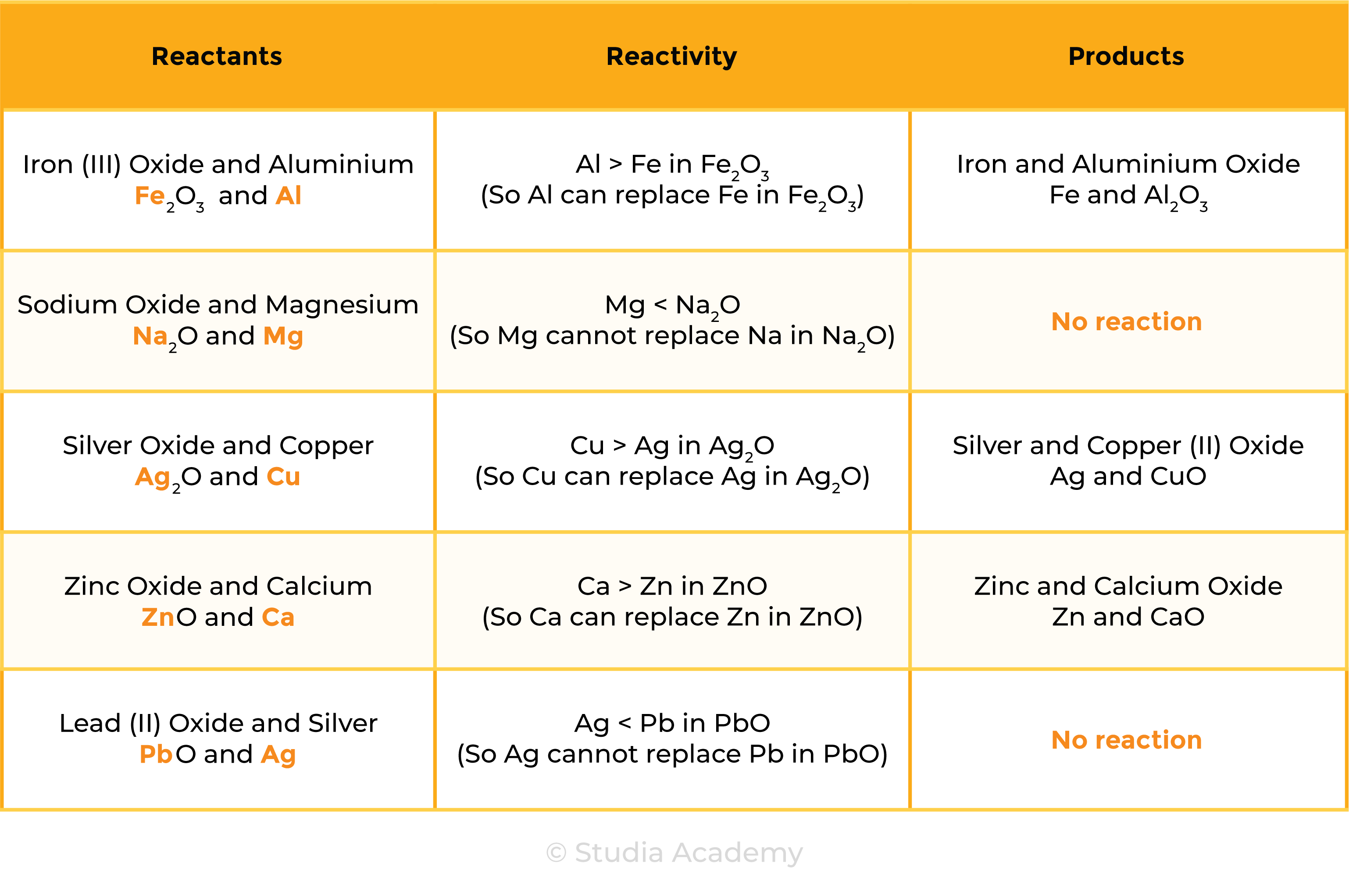
2.4.2 Understand how metals can be arranged in a reactivity series based on their displacement reactions between:
The reactivity of metal decreases going down the reactivity series
REACTION 1 METAL + METAL OXIDE
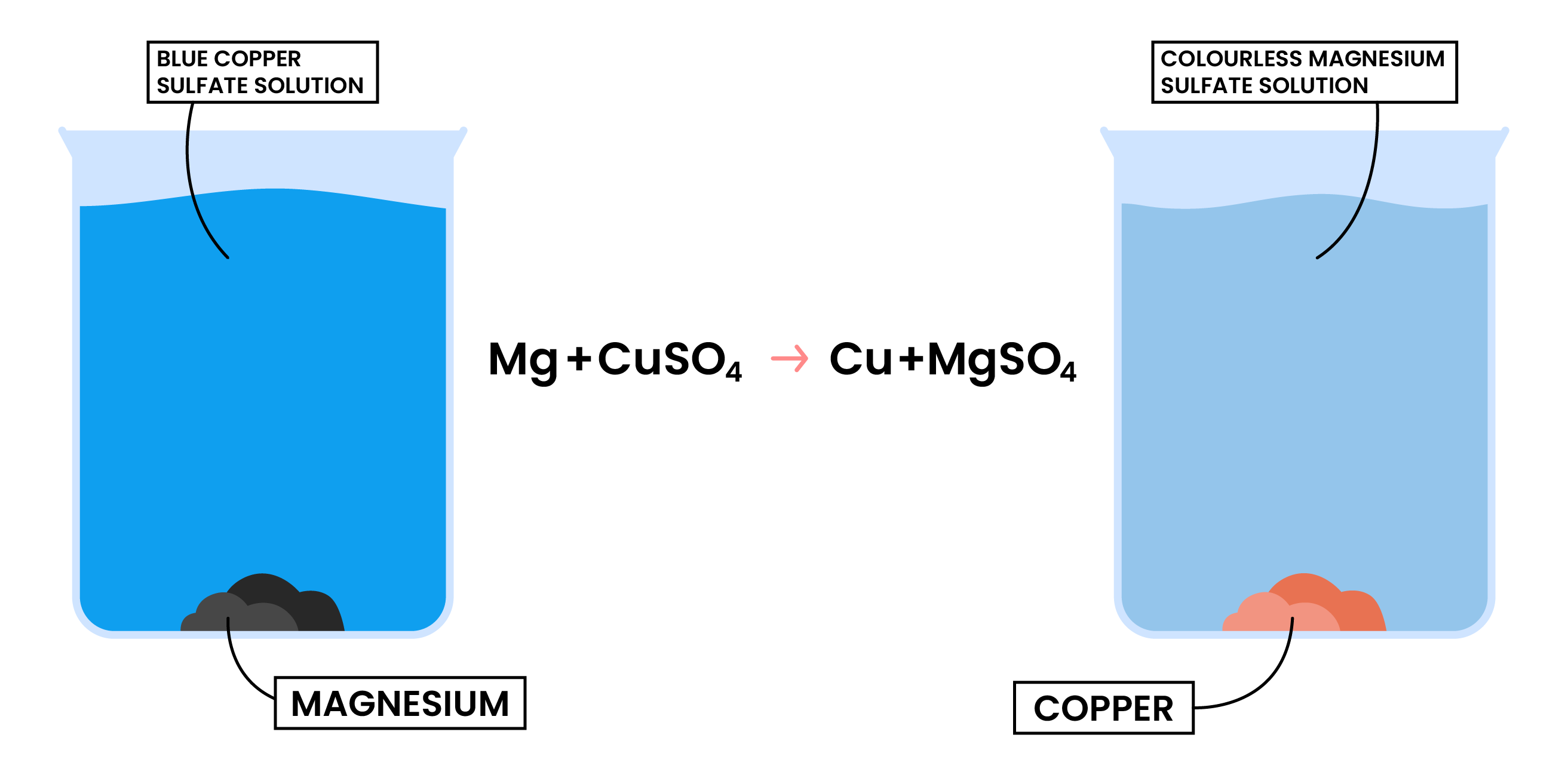
REACTION 2 METAL + AQUEOUS SOLUTION OF METAL SALTS
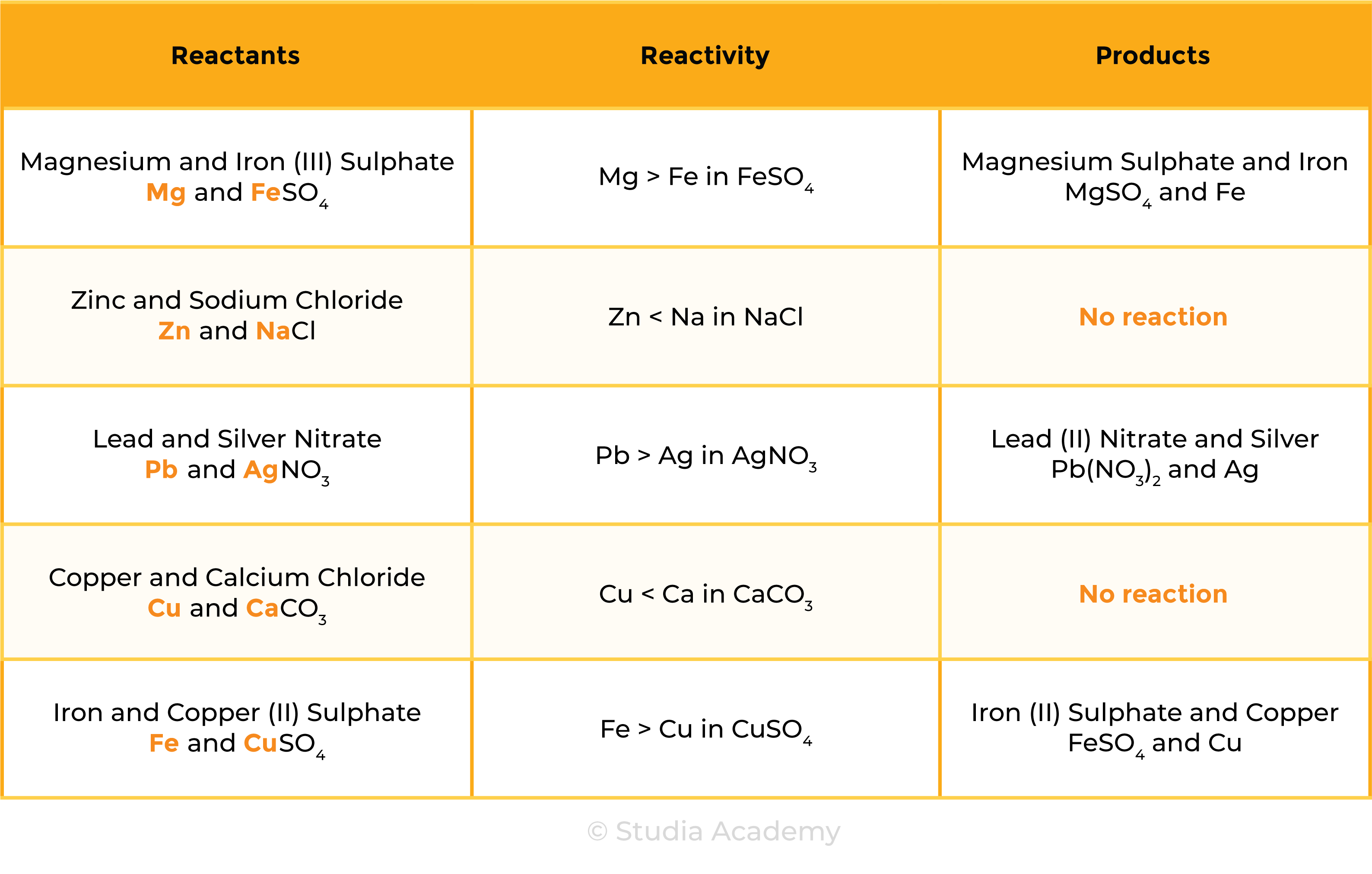
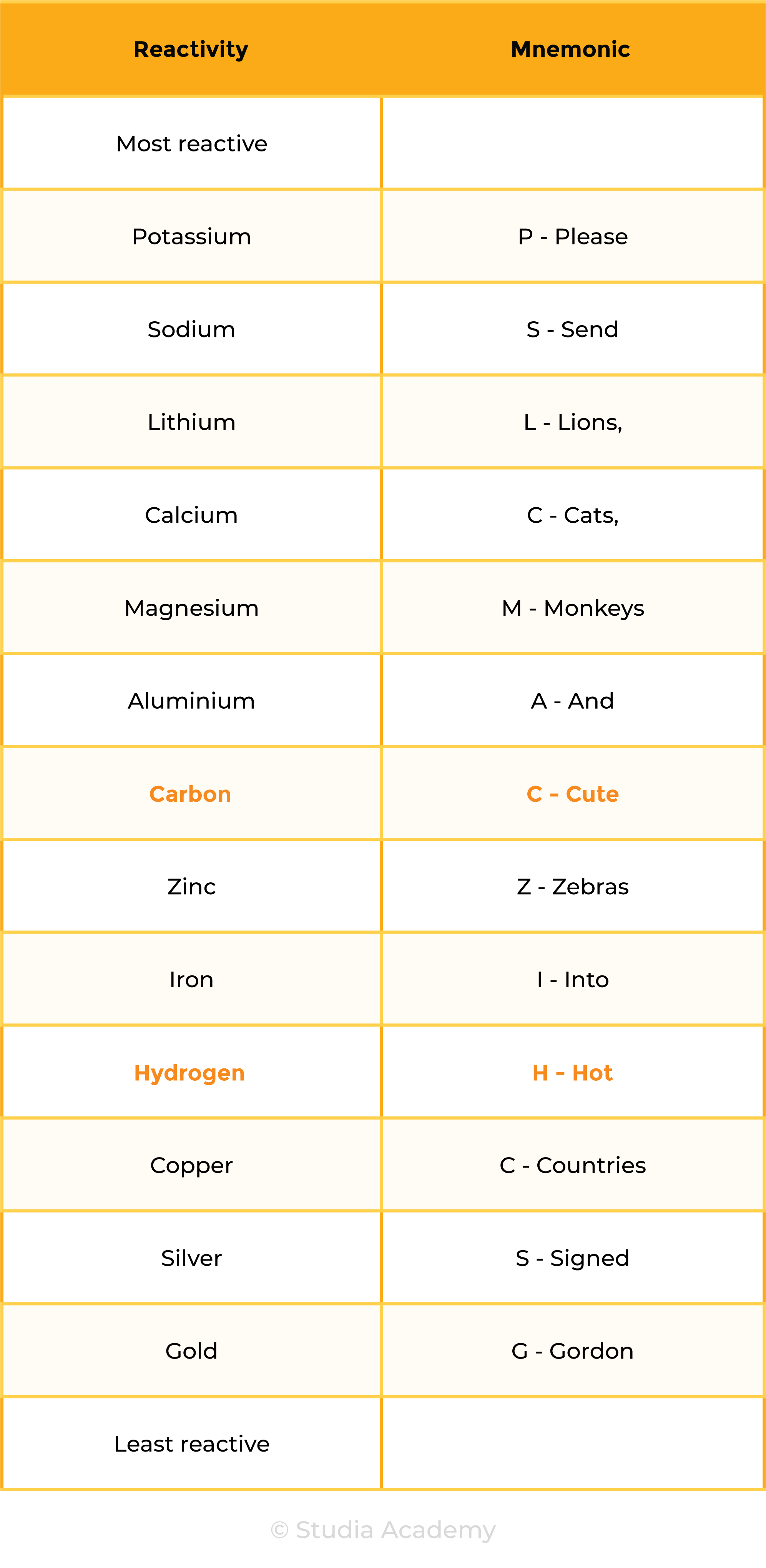
2.4.3 Know the order of reactivity of these metals: potassium, sodium, lithium, calcium, magnesium, aluminium, zinc, iron, copper, silver, gold
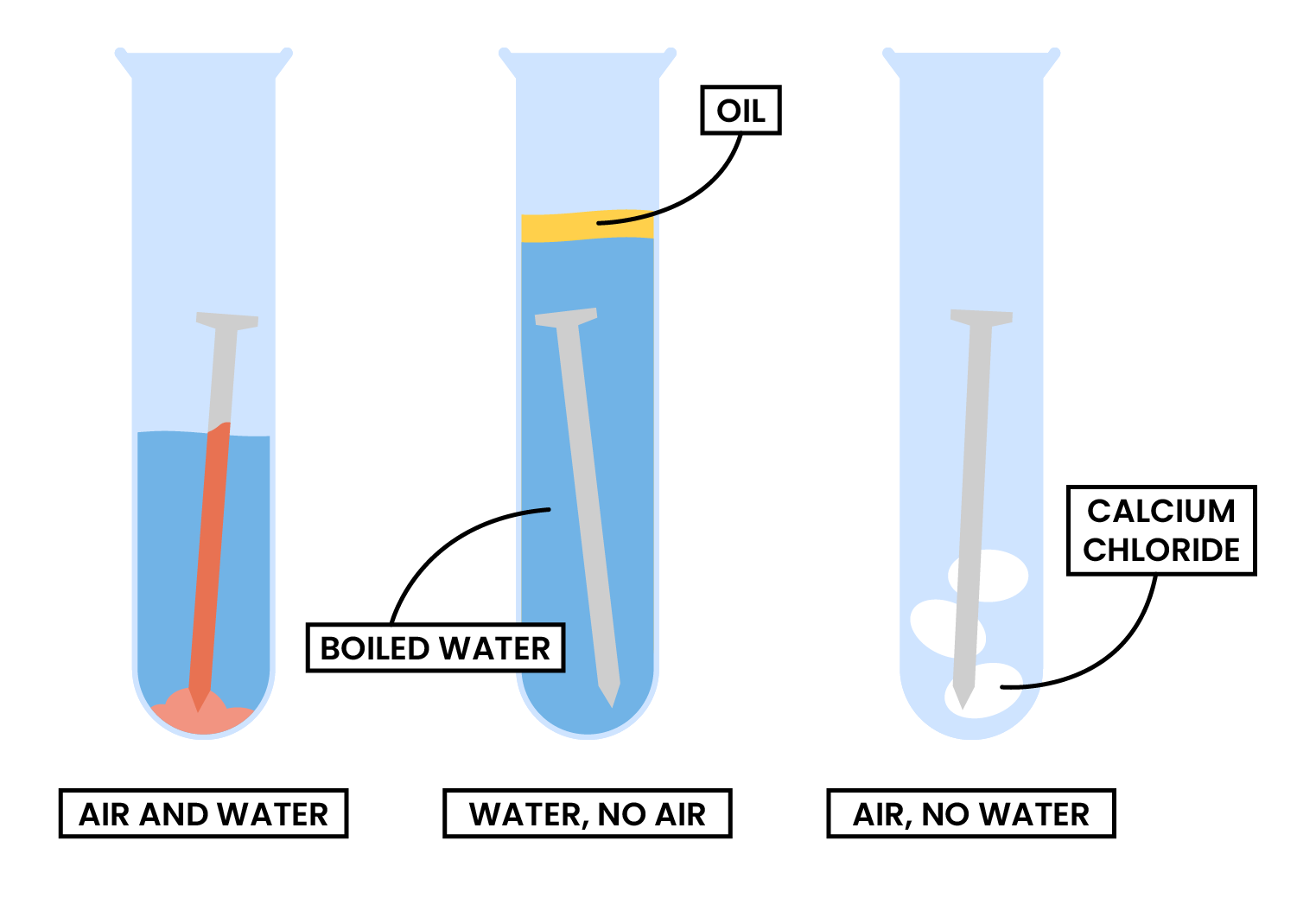
2.4.4 Know the conditions under which iron rusts
RUSTING OF IRON
Iron + Water + Oxygen → Hydrated Iron(III) Oxide
4Fe (s) + 3O2 (g) + xH2O (l) → 2Fe2O3・xH2O (s)
2.4.5 Understand how the rusting of iron may be prevented by:
METHOD 1 BARRIER METHOD
METHOD 2 GALVANISING
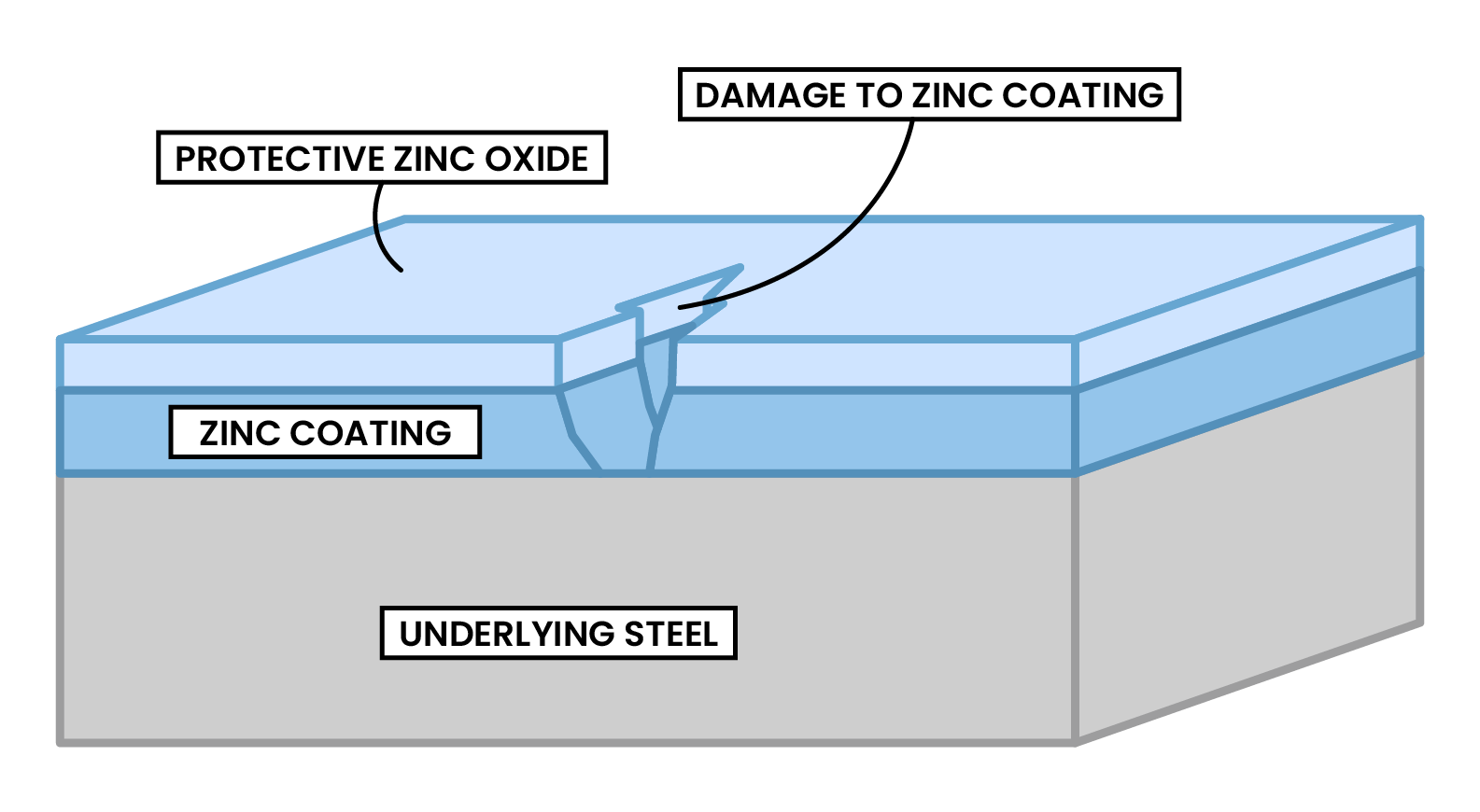
METHOD 3 SACRIFICIAL CORROSION
Example: ships’ hull
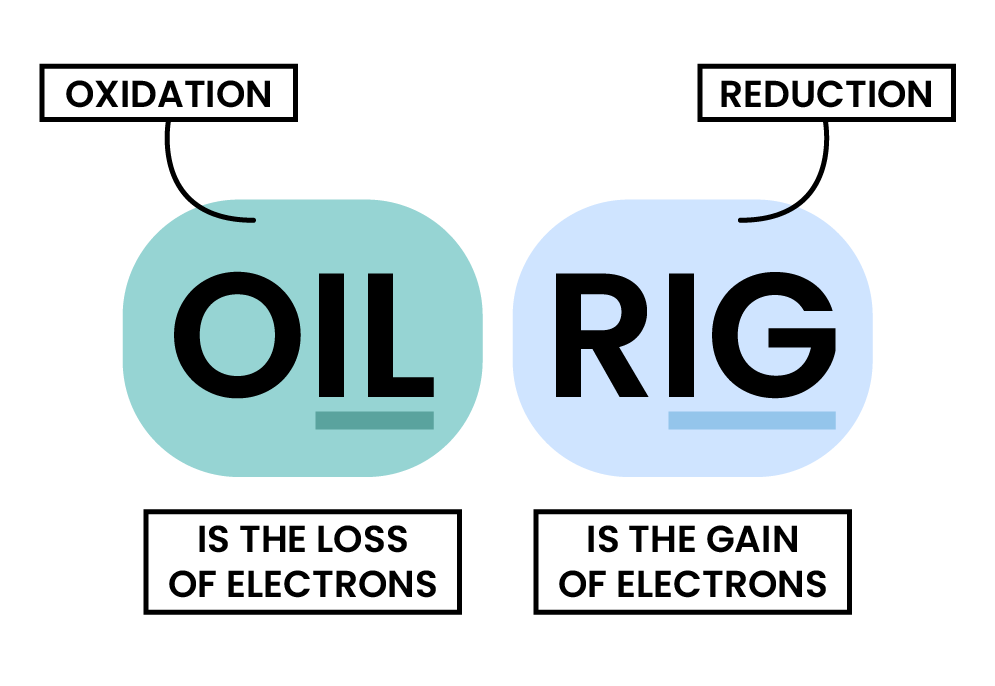
2.4.6 Understand the terms:
in terms of gain or loss of oxygen and loss or gain of electrons.
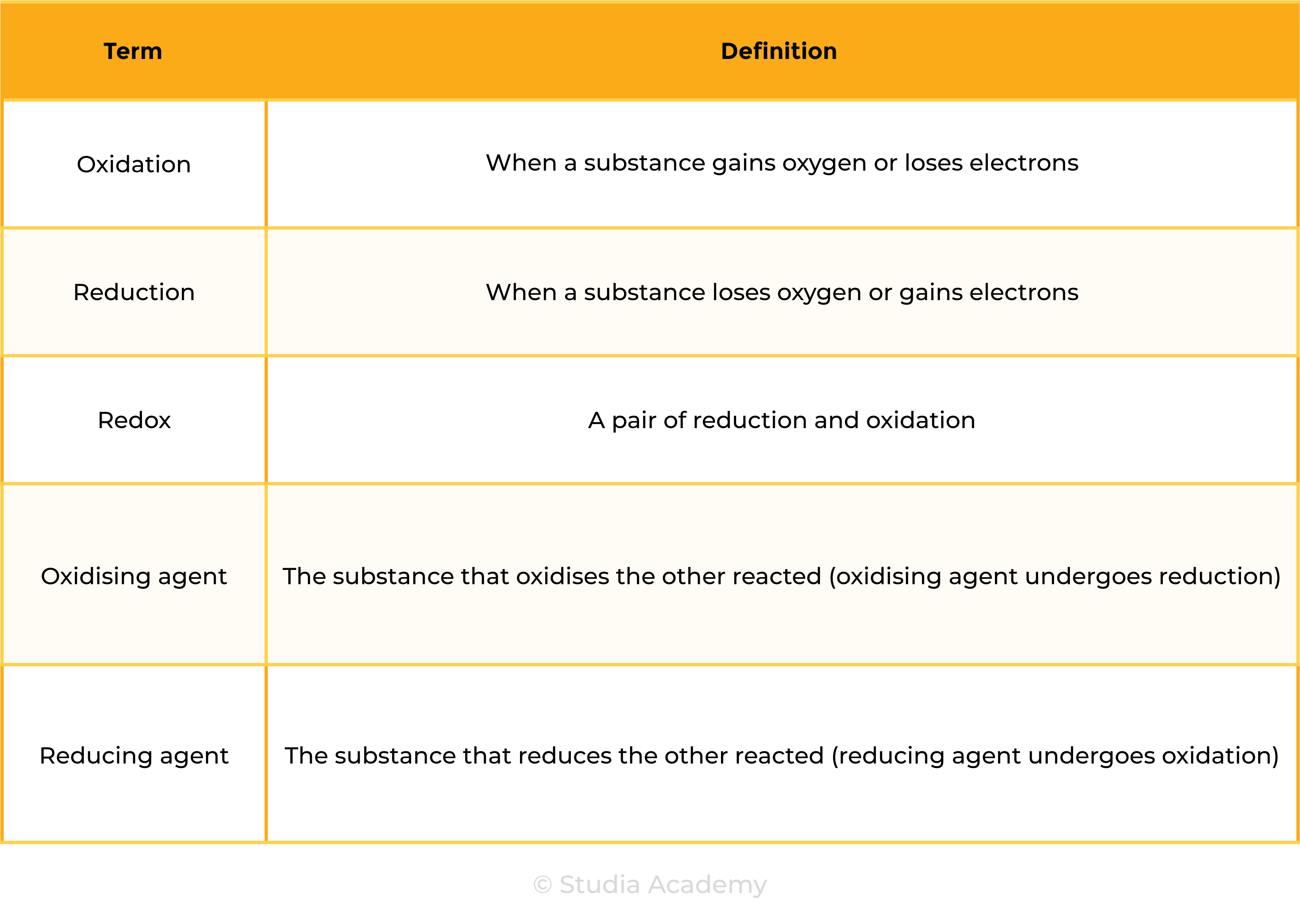
REDOX REACTION (IN TERMS OF OXYGEN)

REDOX REACTION (IN TERMS OF ELECTRON)
Zn → Zn2+ + 2e–
Cu2++ 2e– → Cu
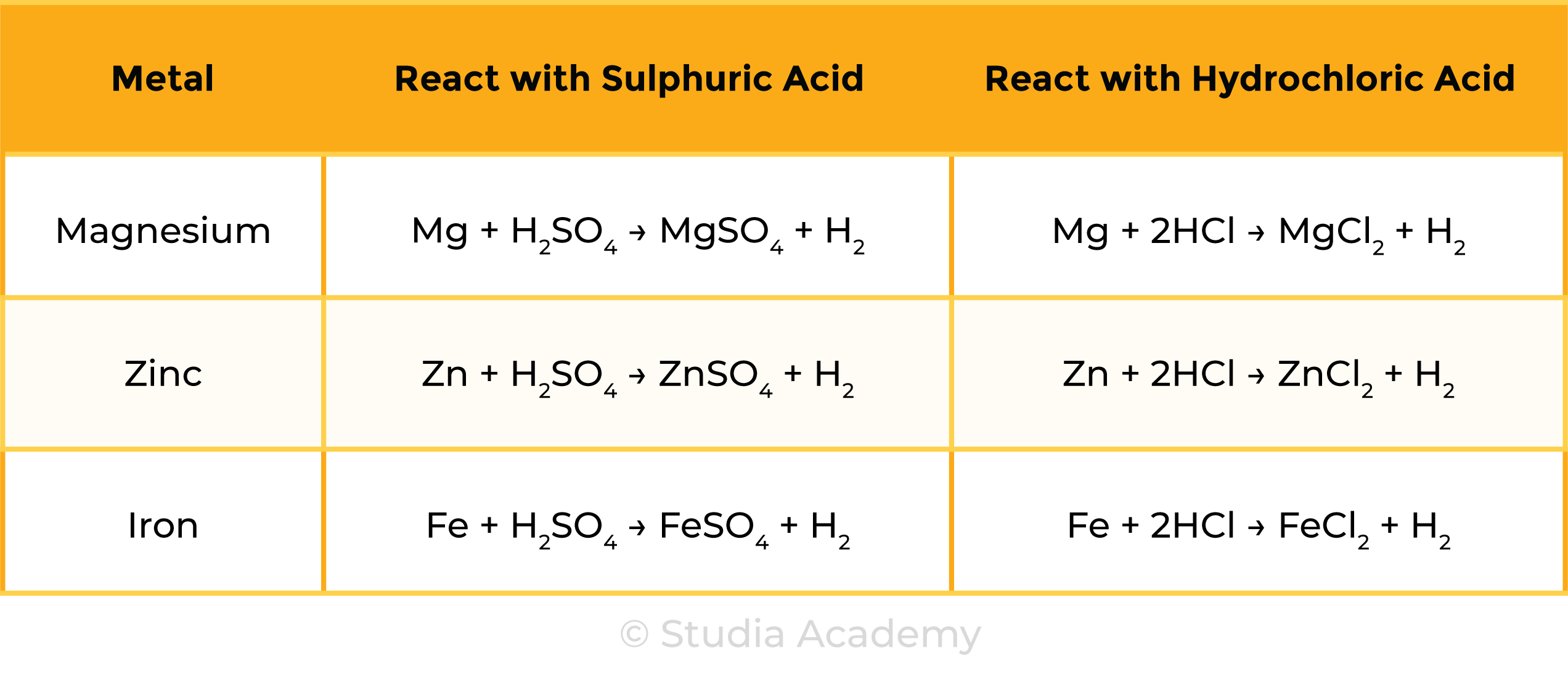
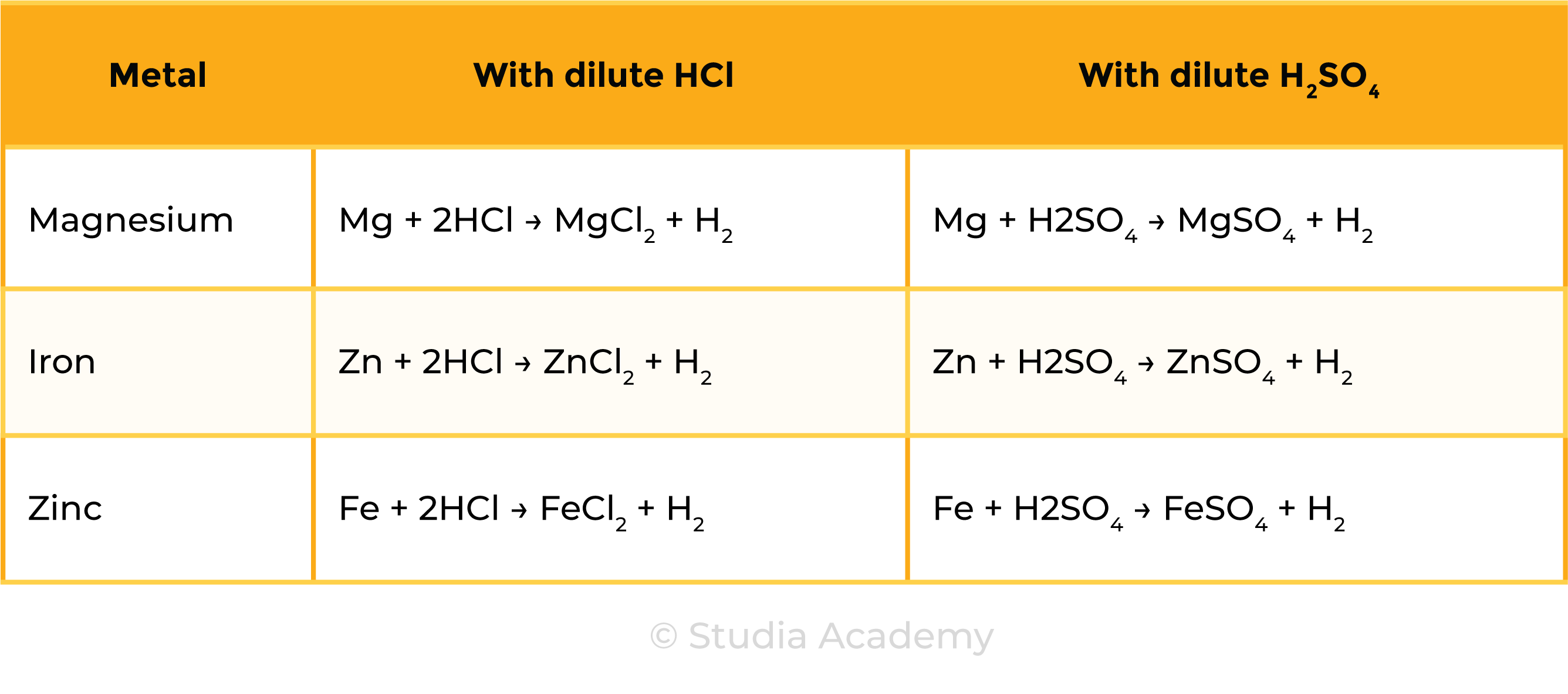
2.4.7 Practical: investigate reactions between dilute hydrochloric and sulfuric acids and metals (e.g. magnesium, zinc and iron)
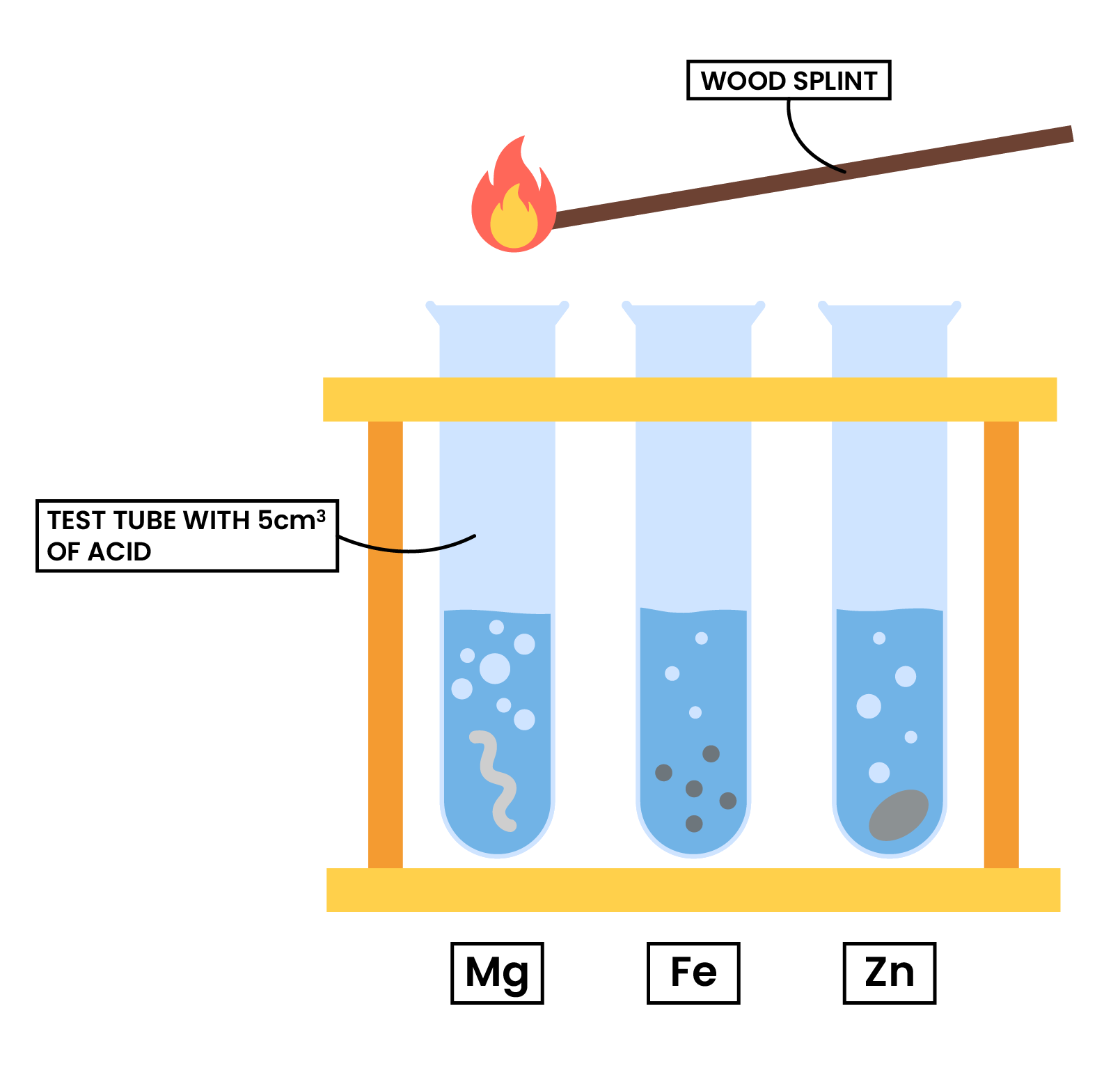
METHODS
Observations
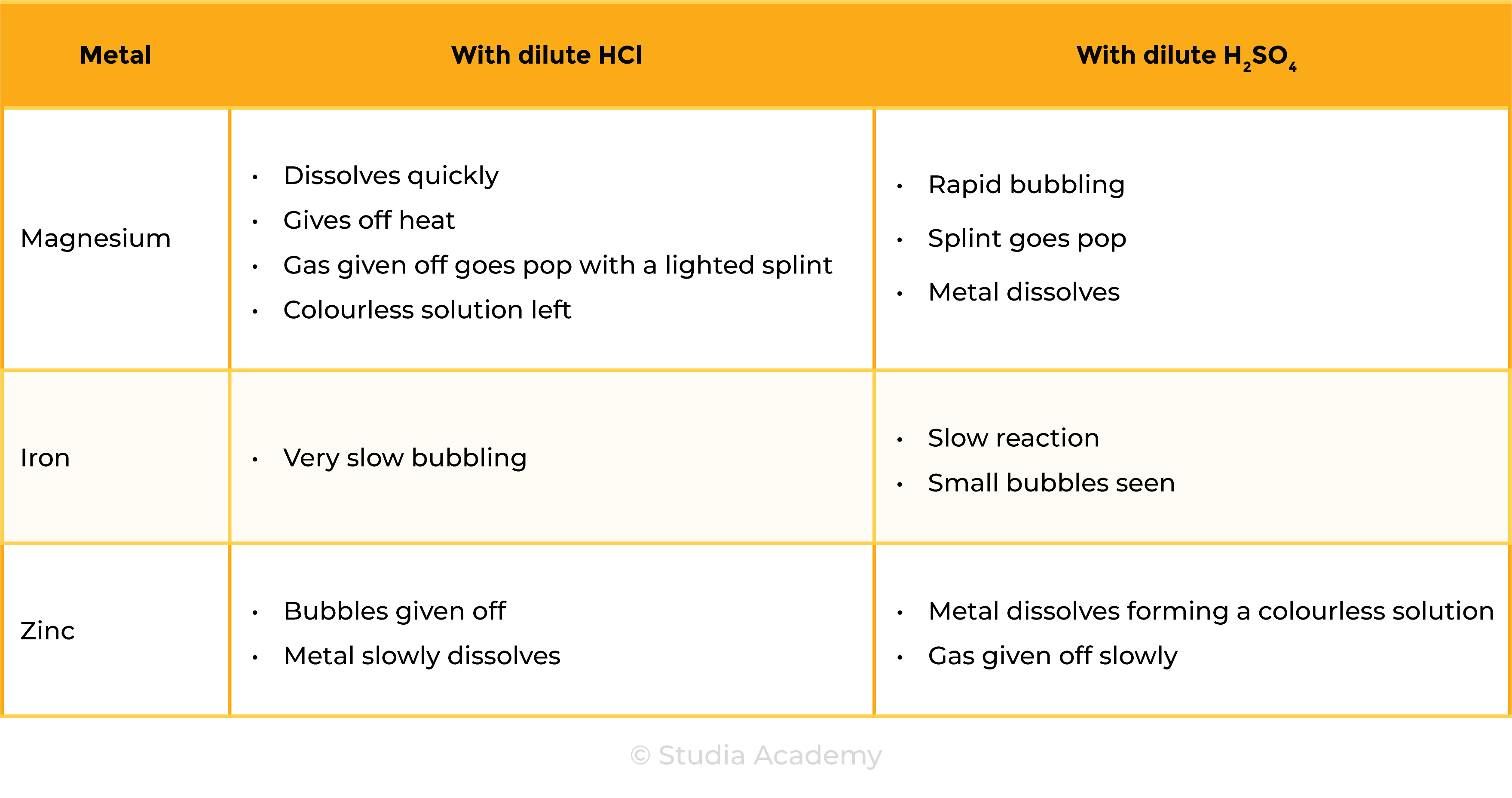
Reactions
CONCLUSION

© 2025 Studia Academy. All rights reserved.