REVISION NOTES
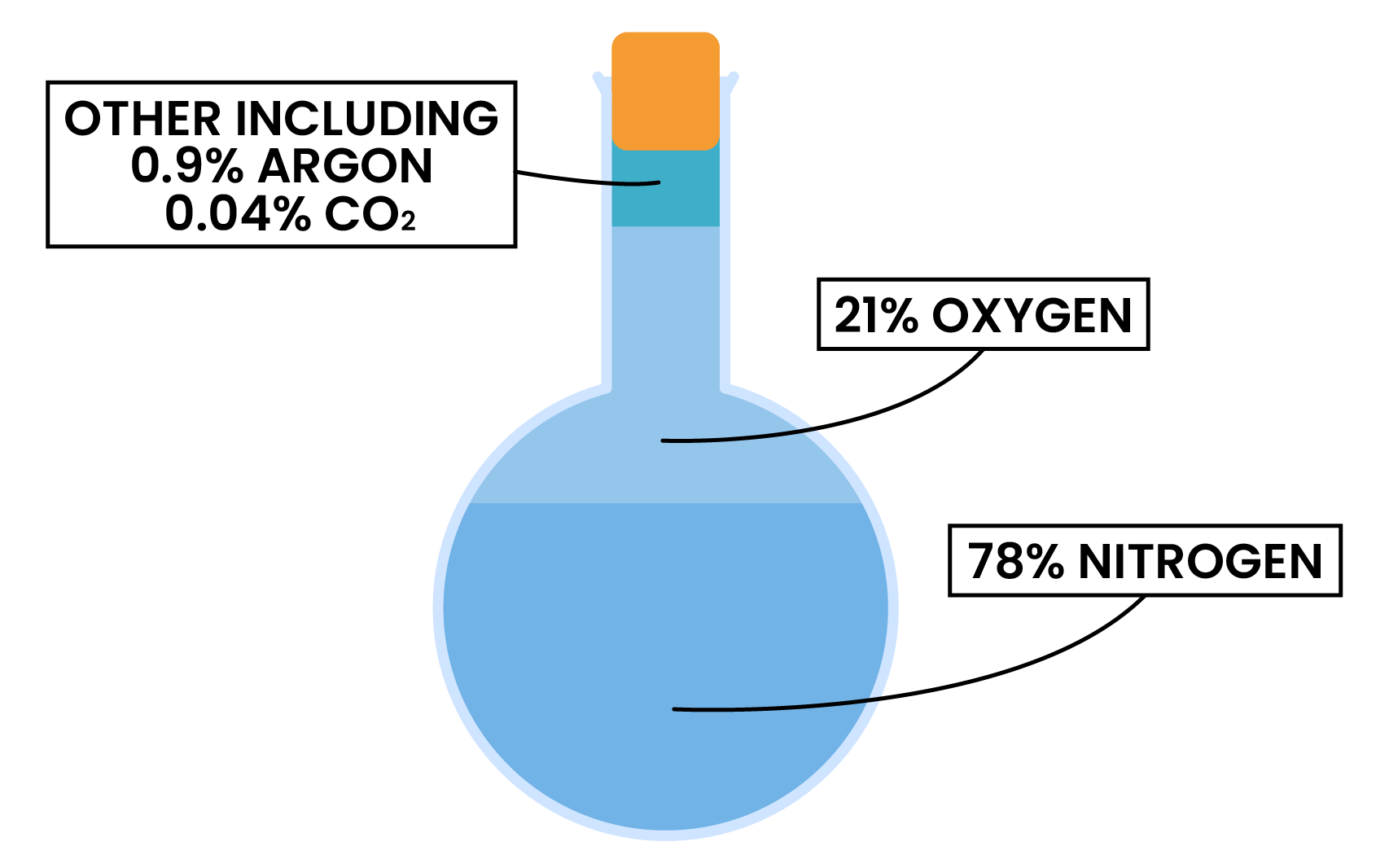
2.3.1 Know the approximate percentages by volume of the four most abundant gases in dry air
PROPORTION OF GASES
2.3.2 Understand how to determine the percentage by volume of oxygen in air using experiments involving the reactions of metals (e.g. iron) and nonmetals
(e.g. phosphorus) with air
The percentage of oxygen in the air can be found by:
Details of experiment will be discussed in 2.3.6
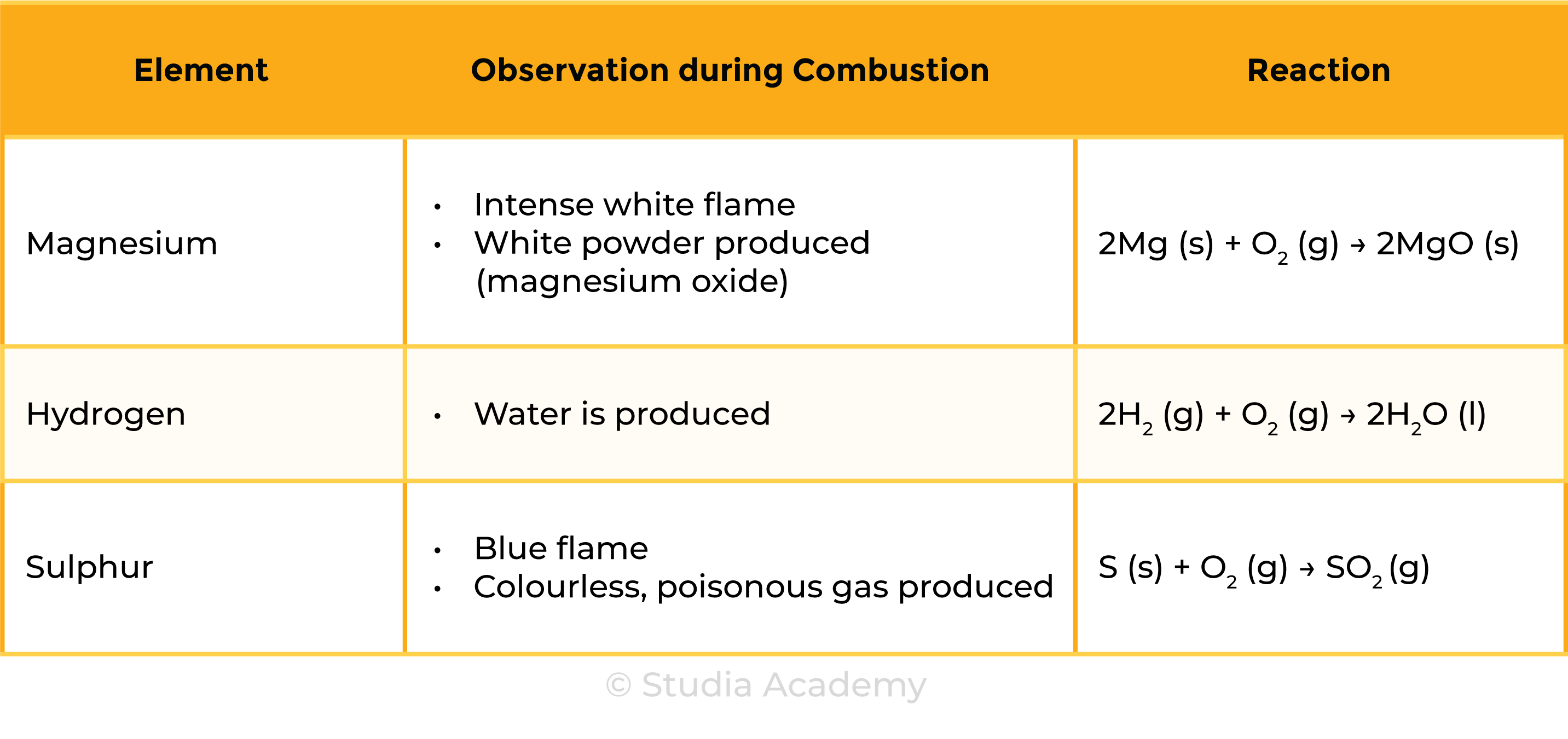
2.3.3 Describe the combustion of elements in oxygen, including magnesium, hydrogen and sulphur
COMBUSTION OF ELEMENTS
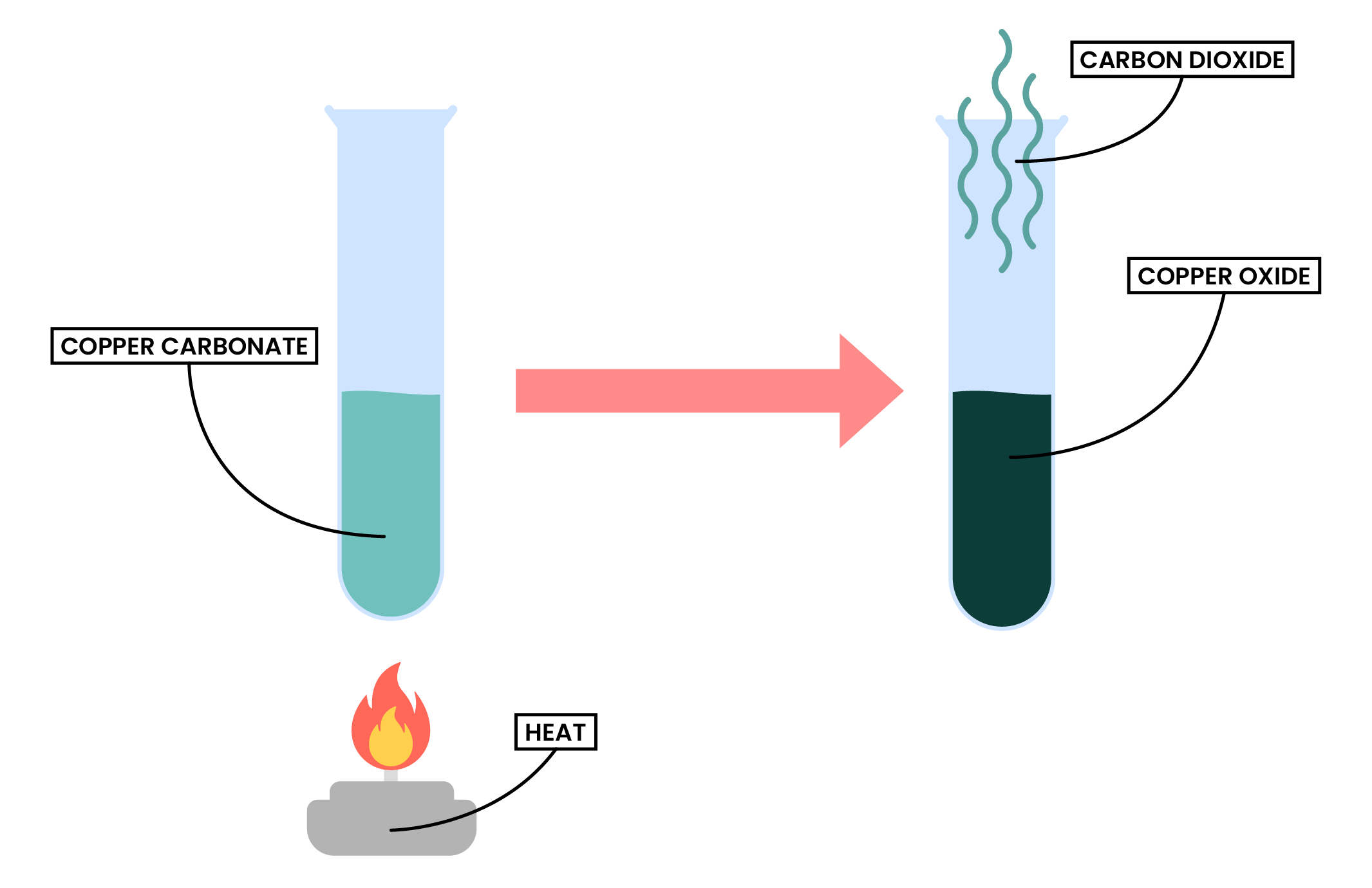
2.3.4 Describe the formation of carbon dioxide from the thermal decomposition of metal carbonates, including copper(II) carbonate
THERMAL DECOMPOSITION
Thermal decomposition of metal carbonate
Metal carbonate → metal oxide + carbon dioxide
Example: copper (II) carbonate
CuCO3 (s) → CuO (s) + CO2 (g)
Copper (II) carbonate → Copper (II) oxide + Carbon dioxide
2.3.5 Know that carbon dioxide is a greenhouse gas and that increasing amounts in the atmosphere may contribute to climate change
ENHANCED GREENHOUSE EFFECT
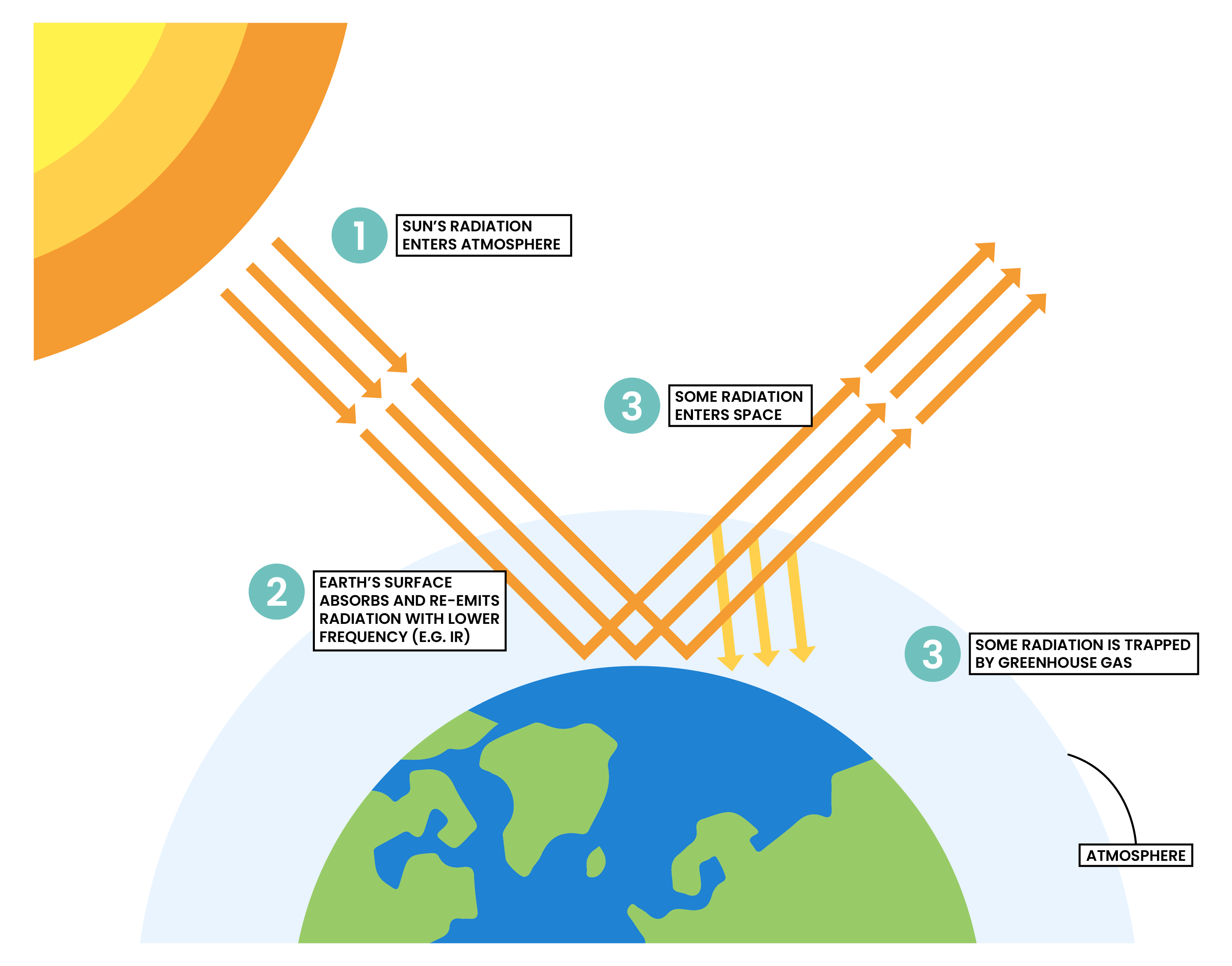
GREENHOUSE GASES
2.3.6 Practical: determine the approximate percentage by volume of oxygen in air using a metal or a non-metal
REACTION 1 COMBUSTION OF PHOSPHORUS
Measurement and calculations
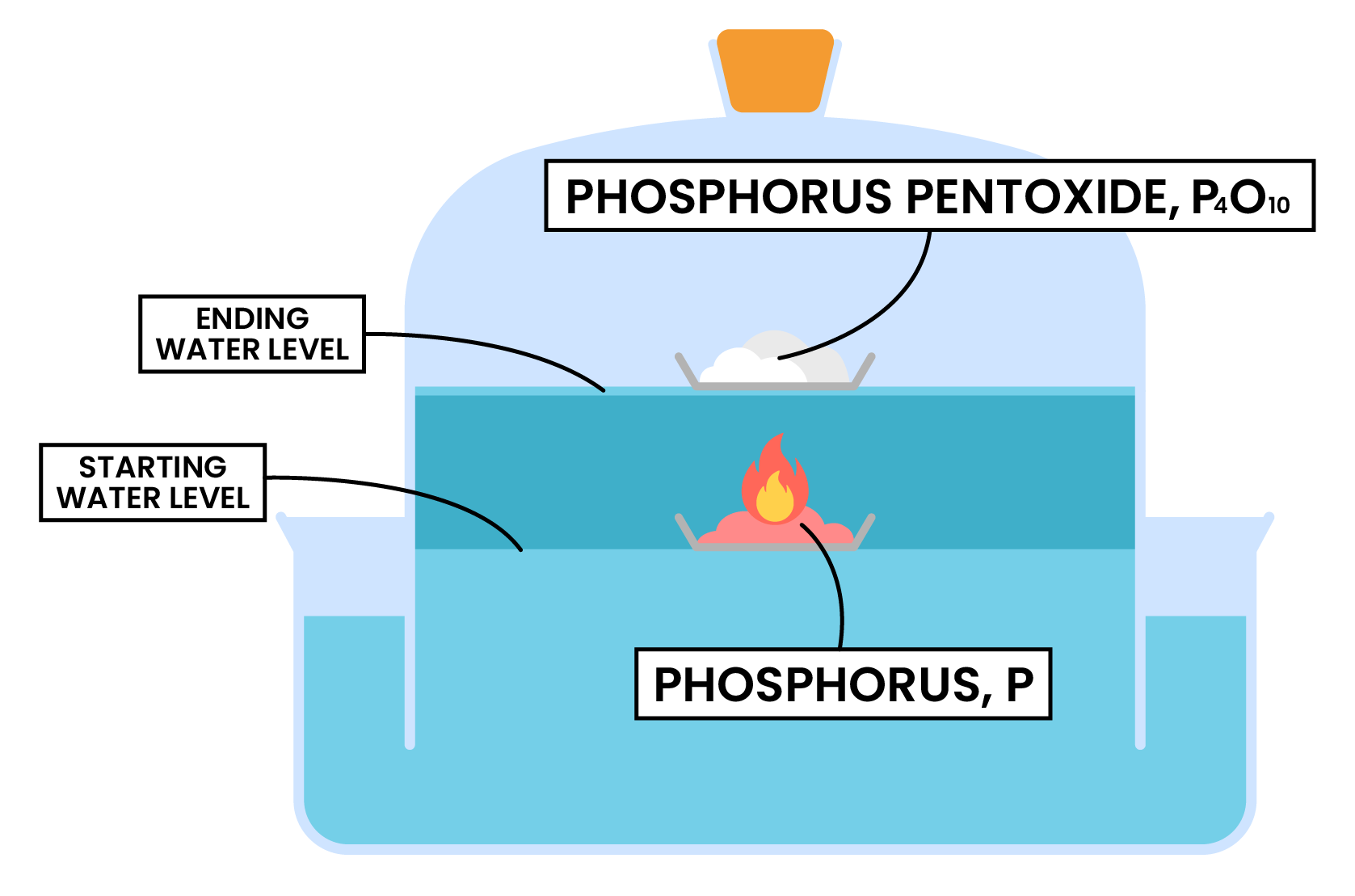
Advantage and disadvantage
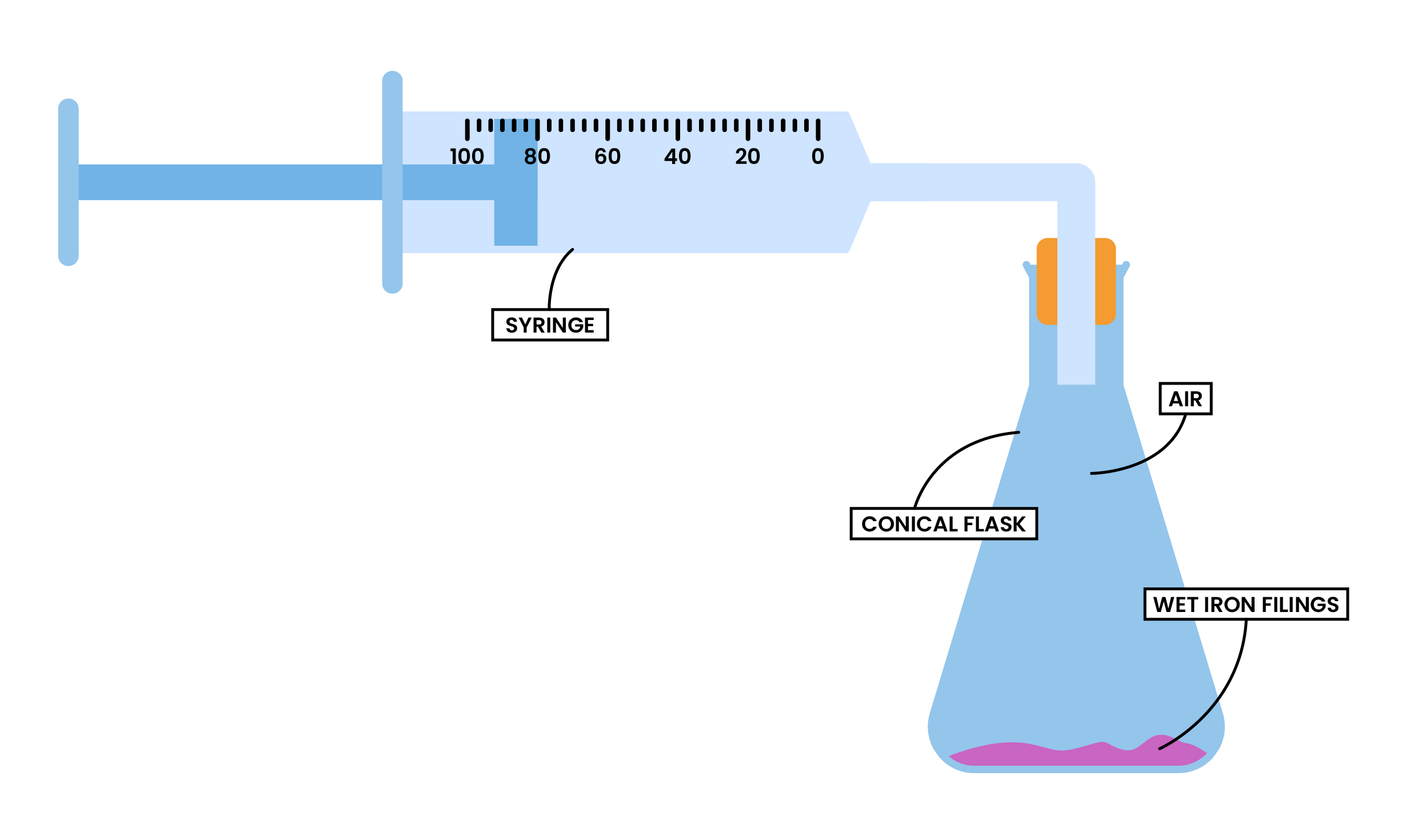
REACTION 2 OXIDATION OF IRON
METHODS
Calculation

© 2025 Studia Academy. All rights reserved.