REVISION NOTES
2.1.1 Understand how the similarities in the reactions of these elements with water provide evidence for their recognition as a family of elements
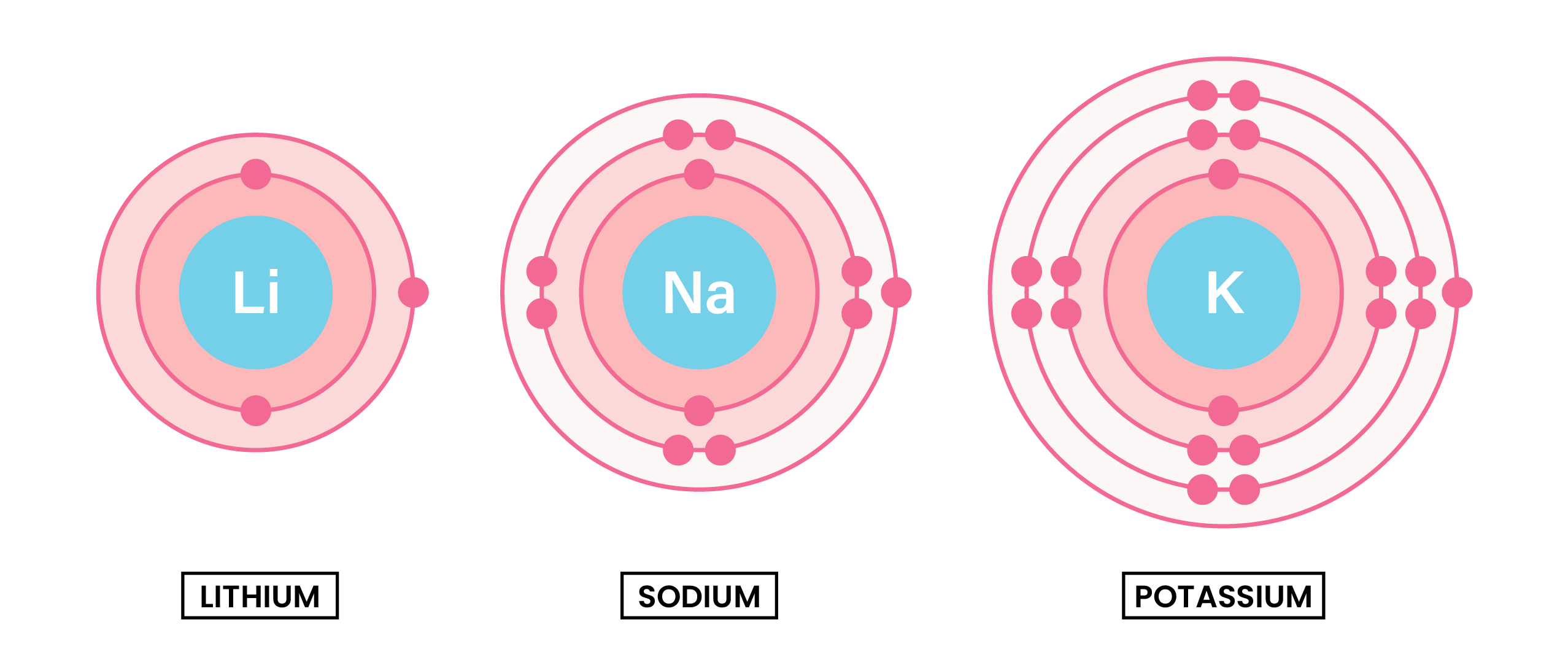
Group 1 metals (alkali metals) are found in the 1st group (column) of the periodic table. They form alkaline solutions when they react with water.
Because they have same number of electrons in the outer shell, they have similar chemical properties.
2.1.2 Understand how the differences between the reactions of these elements with air and water provide evidence for the trend in reactivity in Group 1
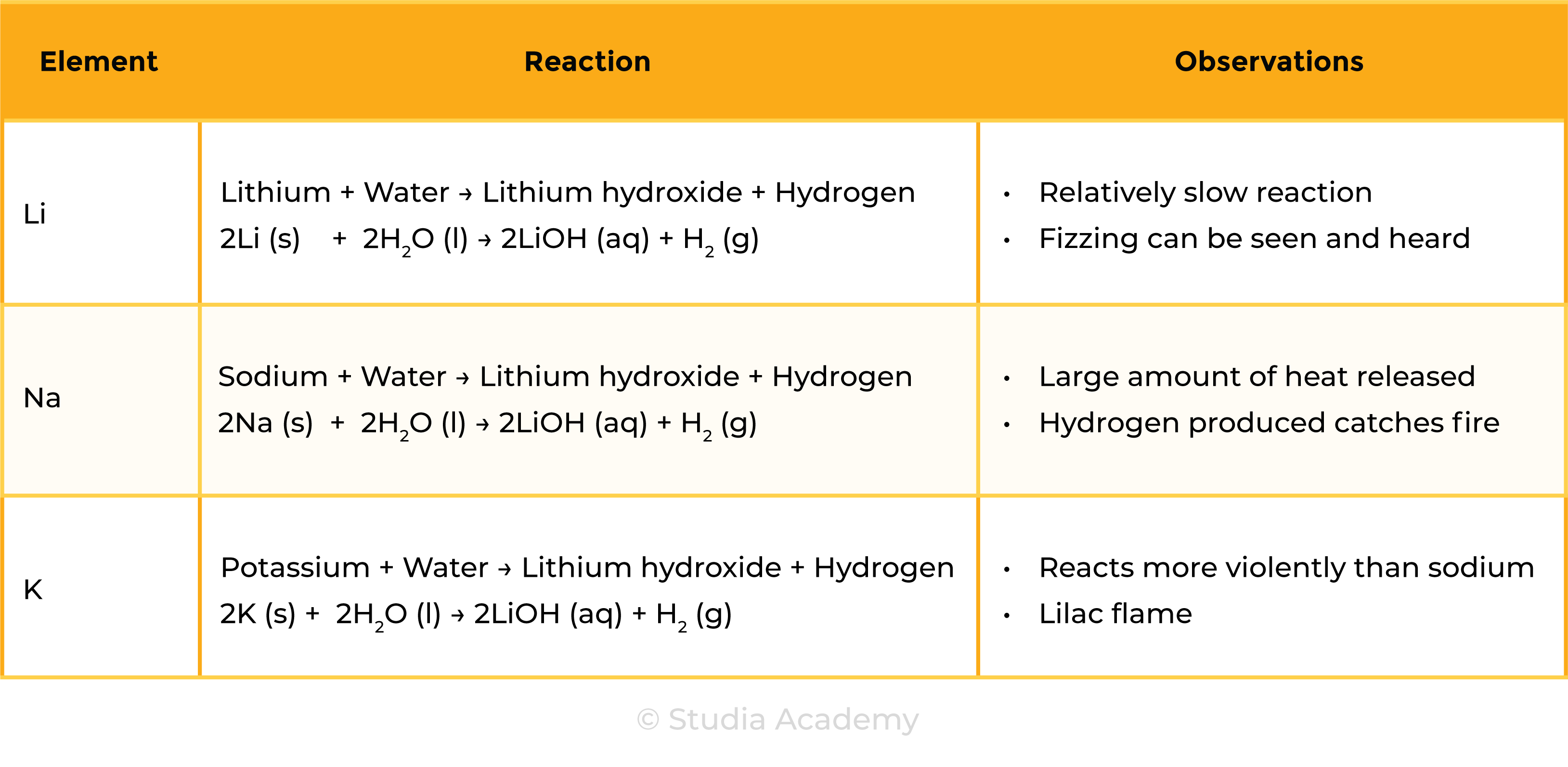
REACTION WITH WATER
Group 1 metal + Water → Metal hydroxide + Hydrogen
2M (s) + 2H2O (l) → 2MOH (aq) + H2 (g)
Observations during Group 1 Metal reaction with Water:
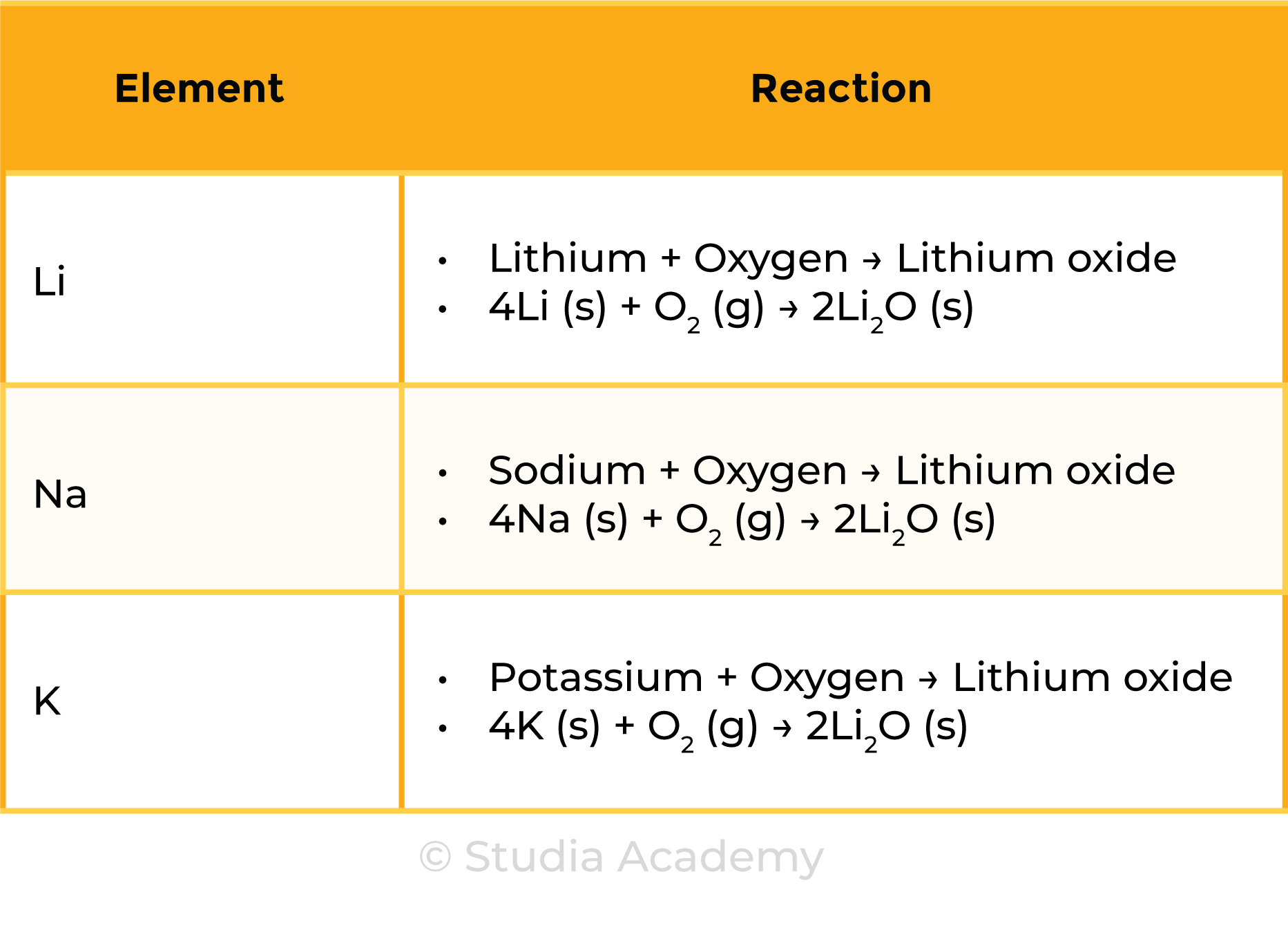
REACTION WITH OXYGEN
2.1.3 Use knowledge of trends in Group 1 to predict the properties of other alkali metals
TRENDS IN CHEMICAL PROPERTIES OF ALKALI METALS
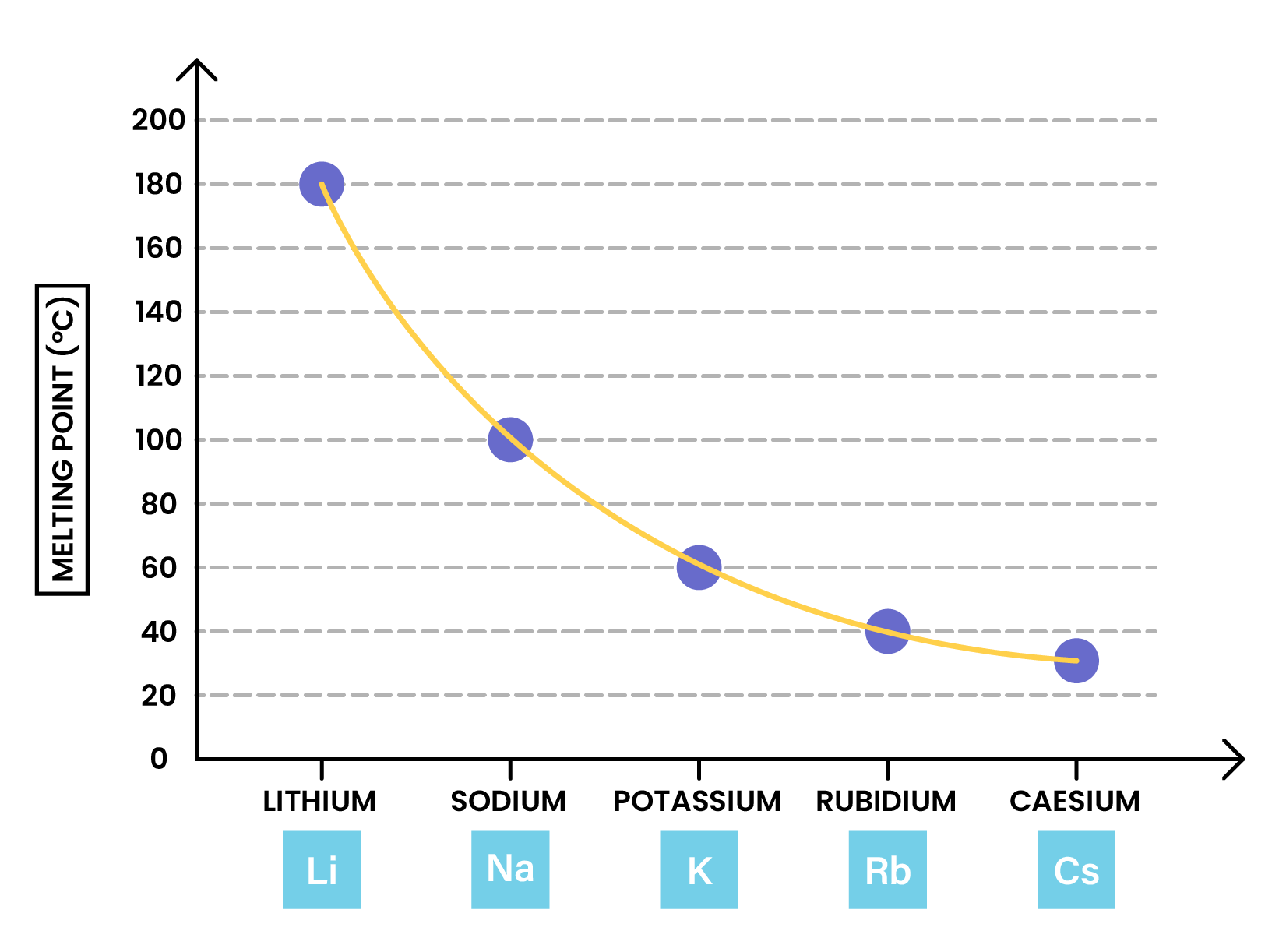
TRENDS IN PHYSICAL PROPERTIES OF ALKALI METALS
PREDICTING PROPERTIES IN GROUP 1
2.1.4C Explain the trend in reactivity in Group 1 in terms of electronic configurations
The reactivity of group 1 increases down the group
EXPLANATION OF TREND IN REACTIVITY

© 2025 Studia Academy. All rights reserved.