REVISION NOTES
IGCSE Edexcel Physics
7.1 Radioactivity
7.1.1 Use the following units: becquerel (Bq), centimetre (cm), hour (h), minute (min) and second (s)

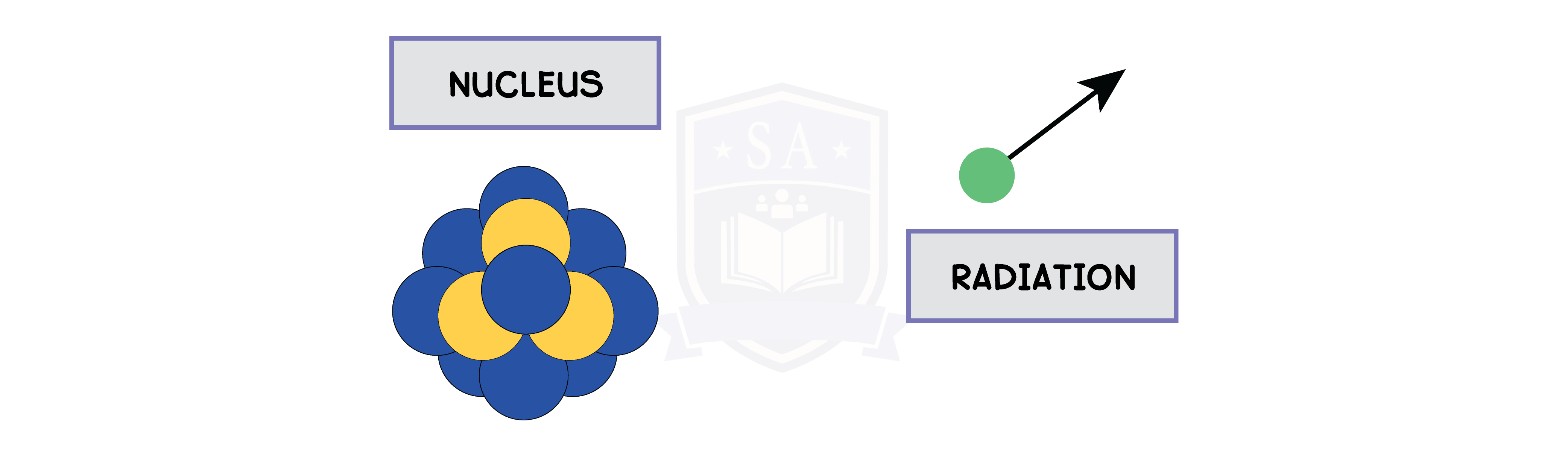
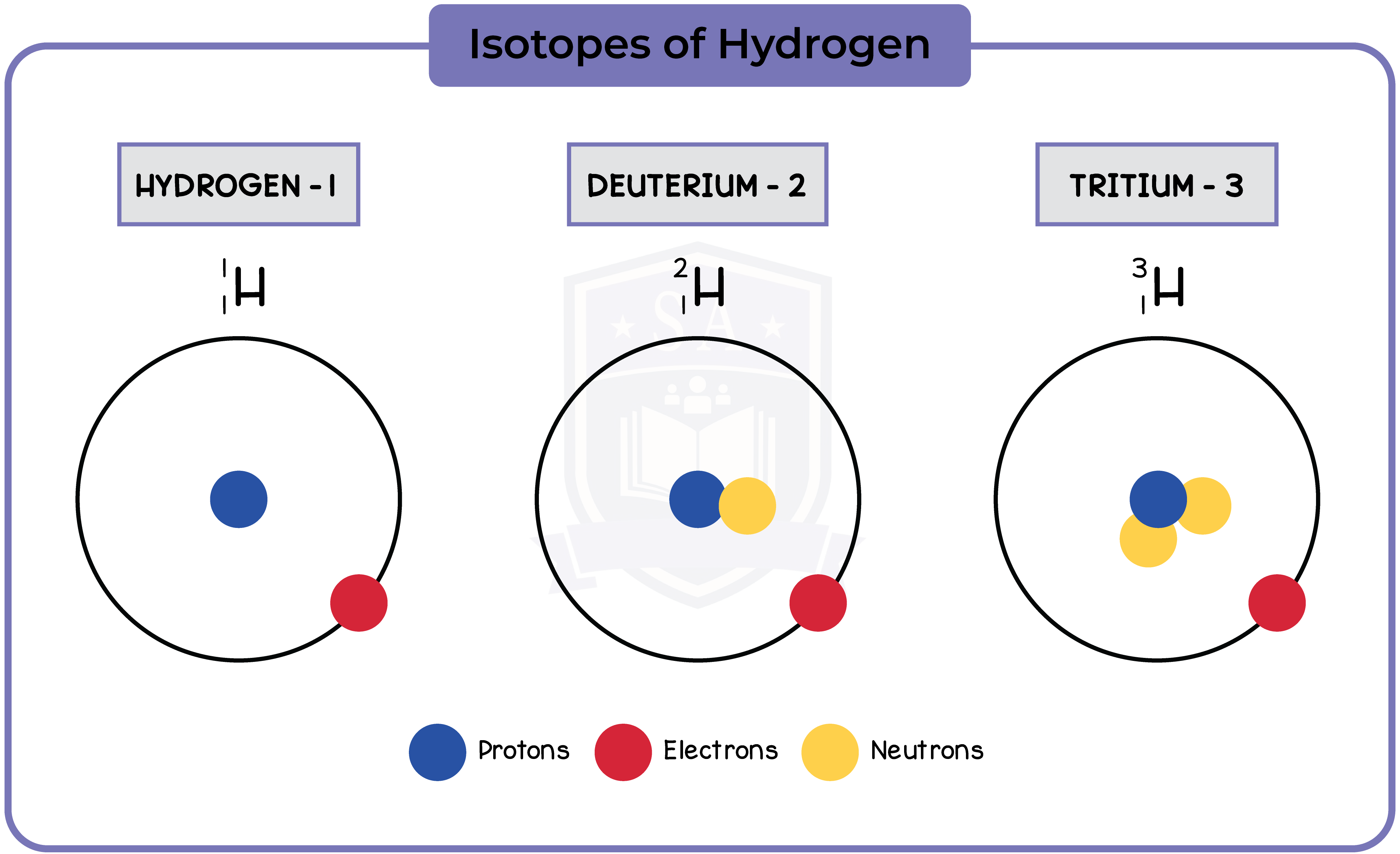
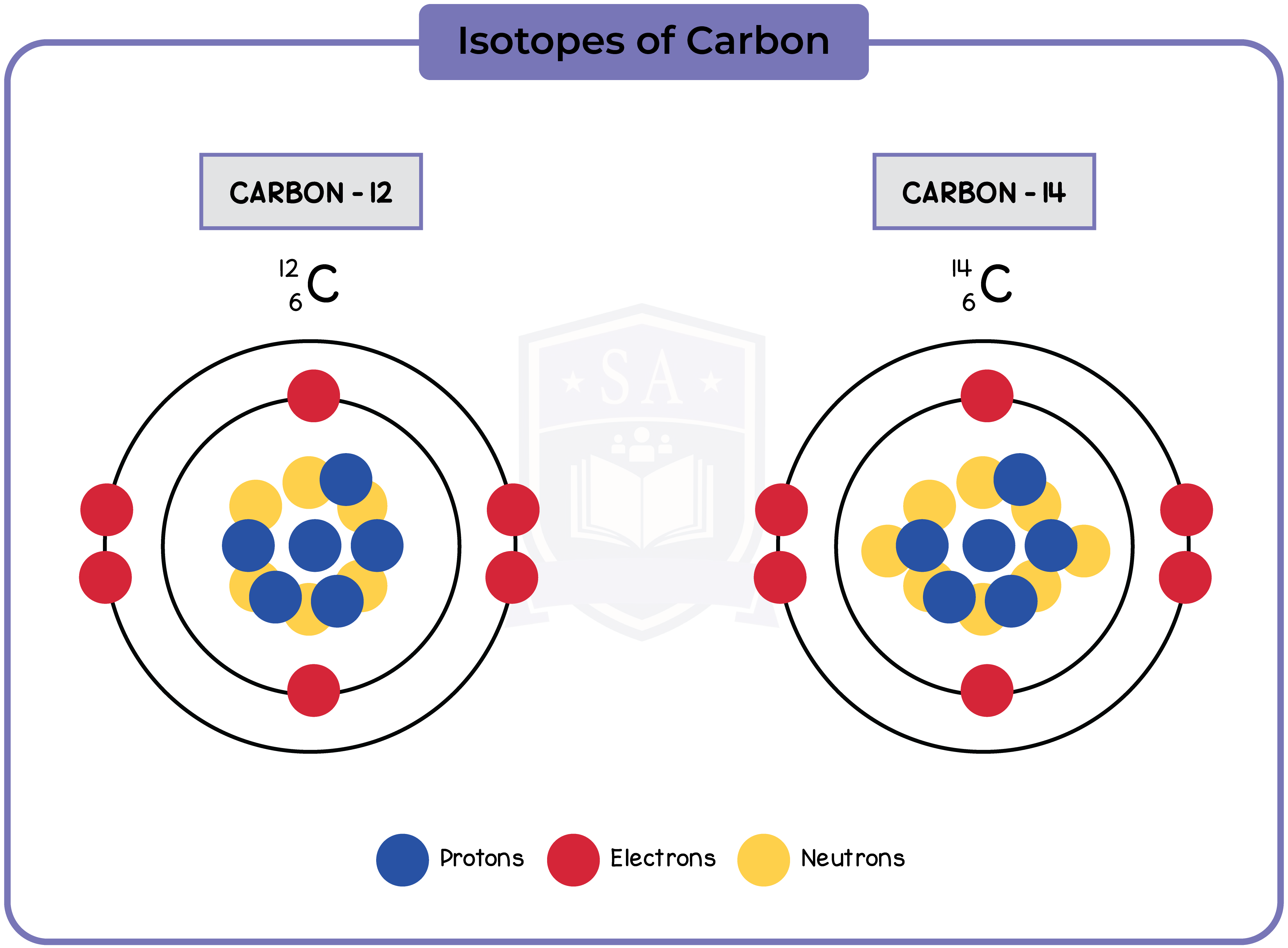
7.1.2 describe the structure of an atom in terms of protons, neutrons and electrons and use symbols such as 146C to describe particular nuclei
- A stable atom has the same amount of protons and electrons
- Protons and neutrons form the nucleus, while electrons orbit the nucleus in shells
- The first shell can have a maximum of 2 electrons, while the rest can have a maximum of 8
- In 146C, 14 is the mass number, and 6 is the atomic number
7.1.3 Know the terms atomic (proton) number, mass (nucleon) number and isotope

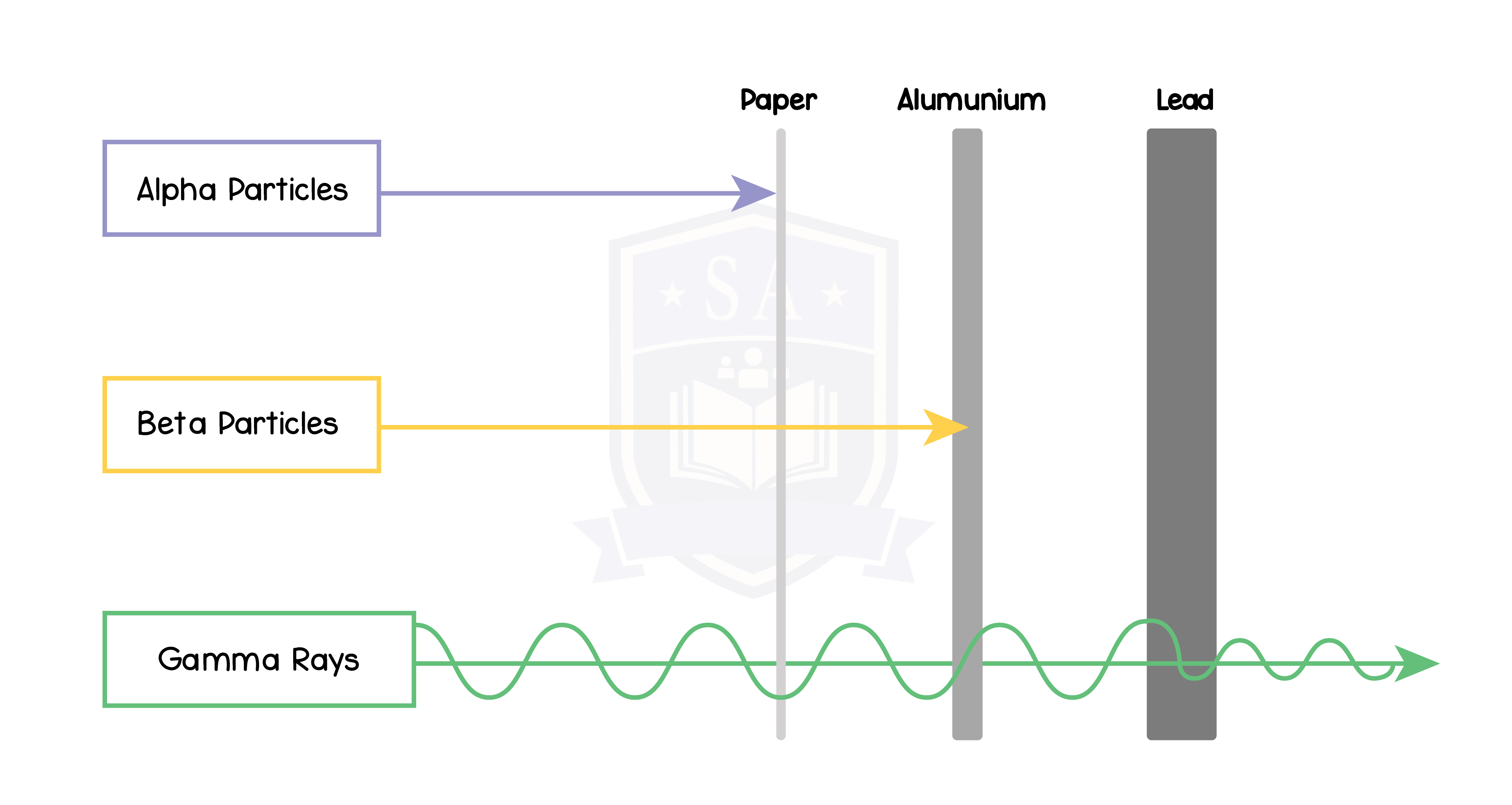
7.1.4 Know that alpha (α) particles, beta (β−) particles, and gamma (γ) rays are ionising radiations emitted from unstable nuclei in a random process

7.1.5 Practical: investigate the penetration powers of different types of radiation using either radioactive sources or simulations

7.1.6 Describe the effects on the atomic and mass numbers of a nucleus of the emission of each of the four main types of radiation (alpha, beta, gamma and neutron radiation)



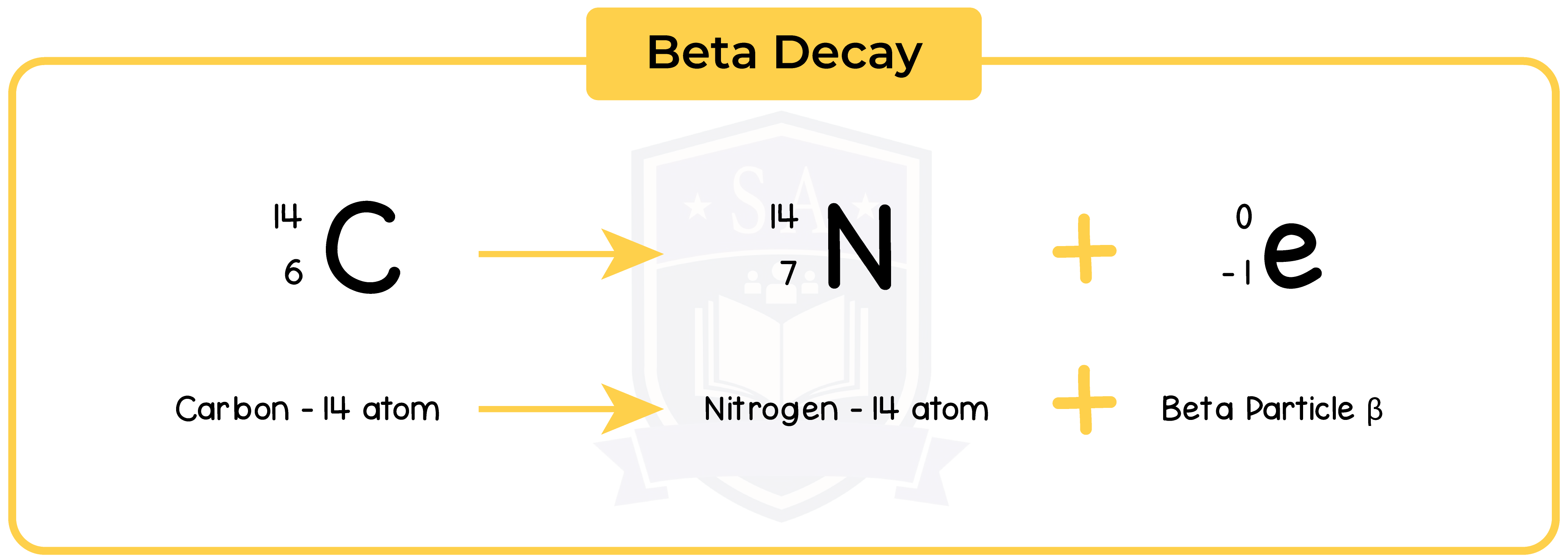

7.1.7 Understand how to balance nuclear equations in terms of mass and charge
7.1.8 Know that photographic film or a Geiger−Müller detector can detect ionising radiations

7.1.9 Explain the sources of background (ionising) radiation from Earth and space
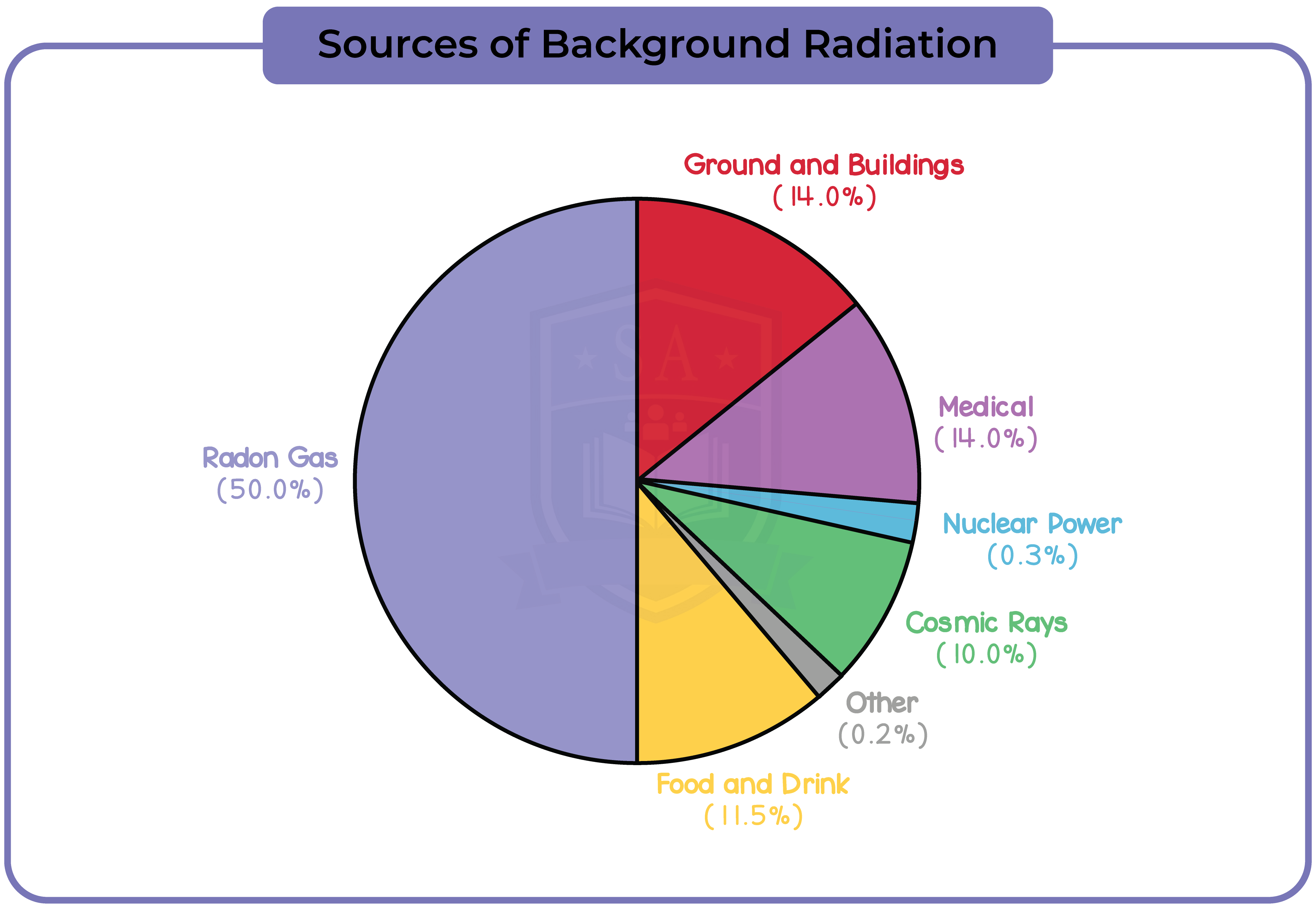
7.1.10 Know that the activity of a radioactive source decreases over a period of time and is measured in becquerels

7.1.11 Know the definition of the term ‘half-life’ and understand that it is different for different radioactive isotopes
7.1.12 Use the concept of the half-life to carry out simple calculations on activity, including graphical methods
7.1.13 Describe uses of radioactivity in industry and medicine
- Medical procedures
- Treating cancer
- Sterilising food
- Sterilising equipment
- Finding the age of certain relics
7.1.14 Describe the difference between contamination and irradiation
- Contamination is the unwanted presence of radioactive sources on non-radioactive objects
- Irradiation is exposing a material to radiation
- Irradiating a material does not make it radioactive
- Irradiation can kill living cells
- Irradiation can be used to sterilise food and medical equipment to kill unwanted microorganisms
7.1.15 Describe the dangers of ionising radiations, including:
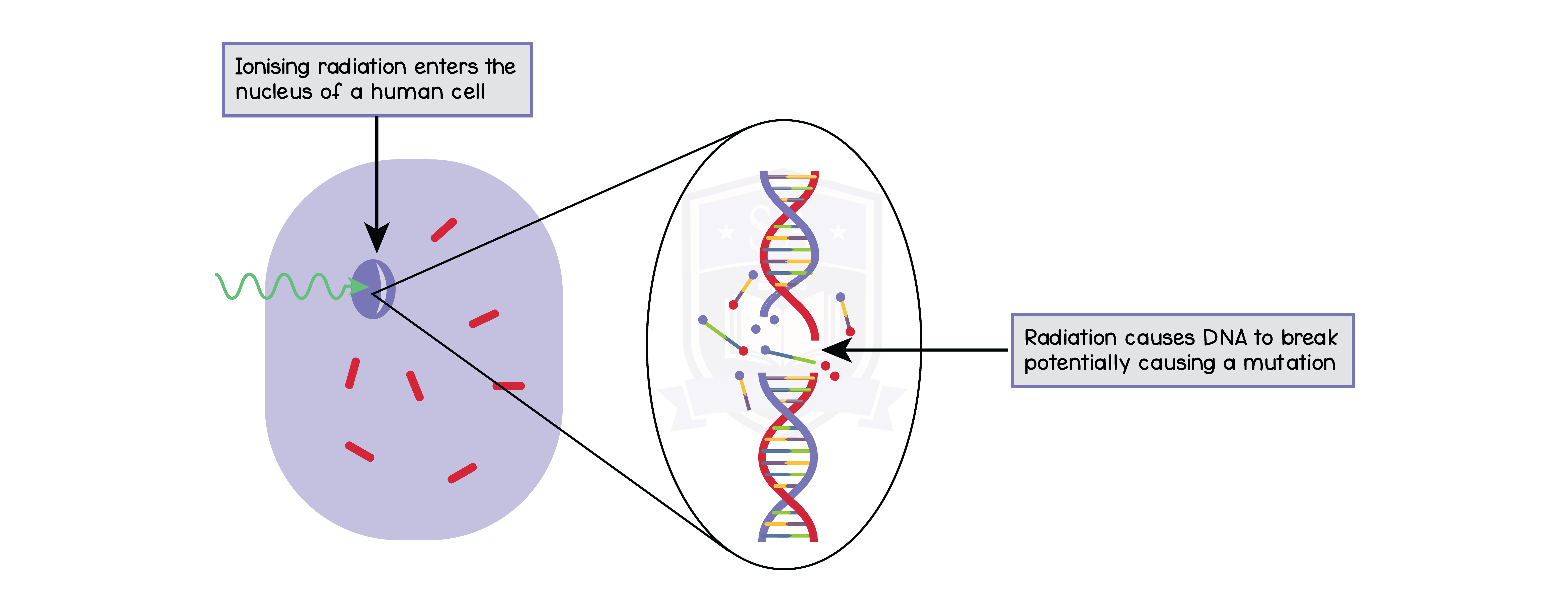
- that radiation can cause mutations in living organisms
- that radiation can damage cells and tissue
- the problems arising from the disposal of radioactive waste and how the associated risks can be reduced

