REVISION NOTES
IGCSE Edexcel Chemistry
1.7 Covalent Bonding
1.7.1 Know that a covalent bond is formed between atoms by the sharing of a pair of electrons
RECALL
- Nonmetals atoms tend to gain electrons to have full electron shells
- When a few nonmetal atoms come together, they tend to share electrons
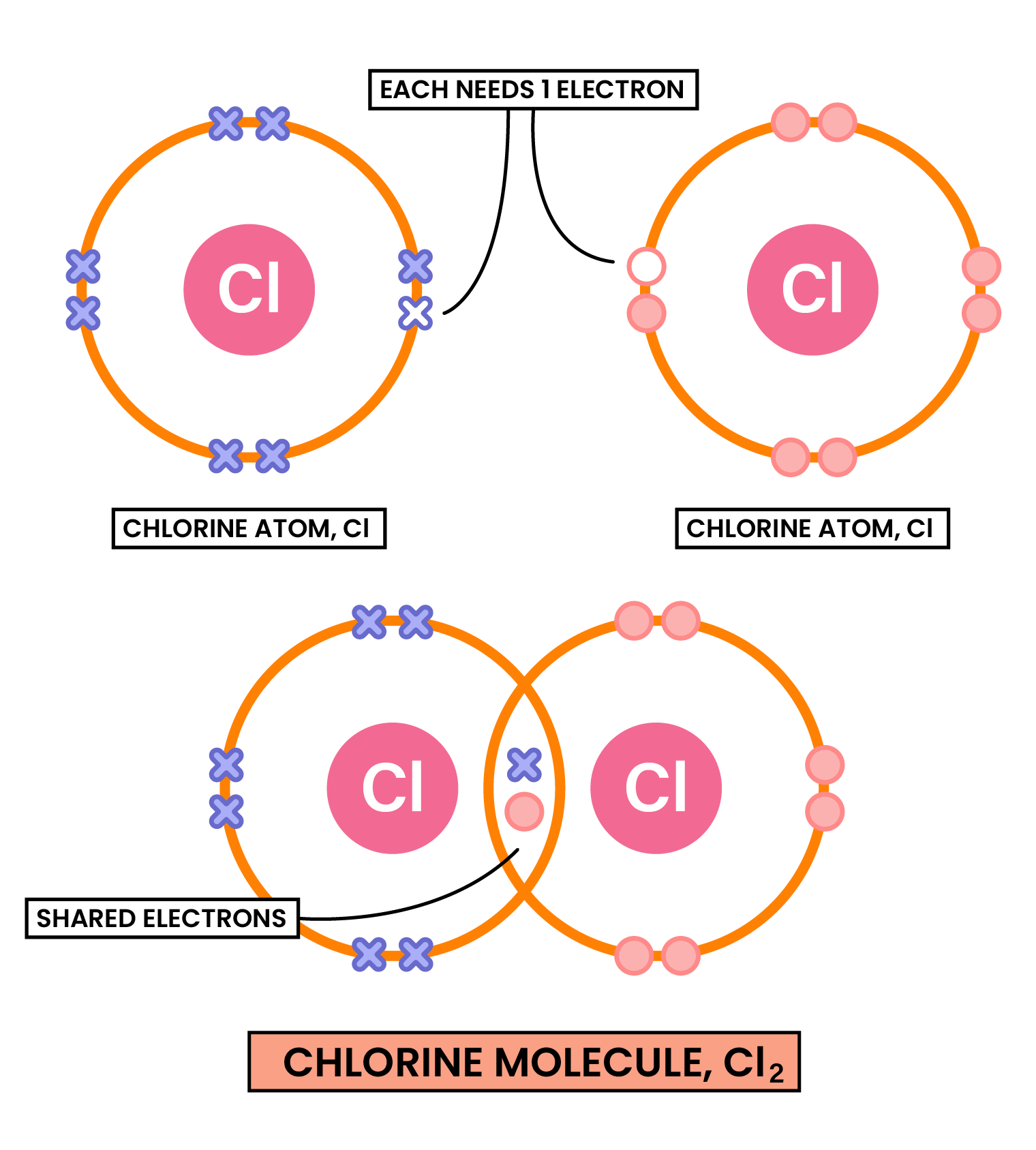
COVALENT COMPOUNDS
- Nonmetal atoms share pairs of electrons
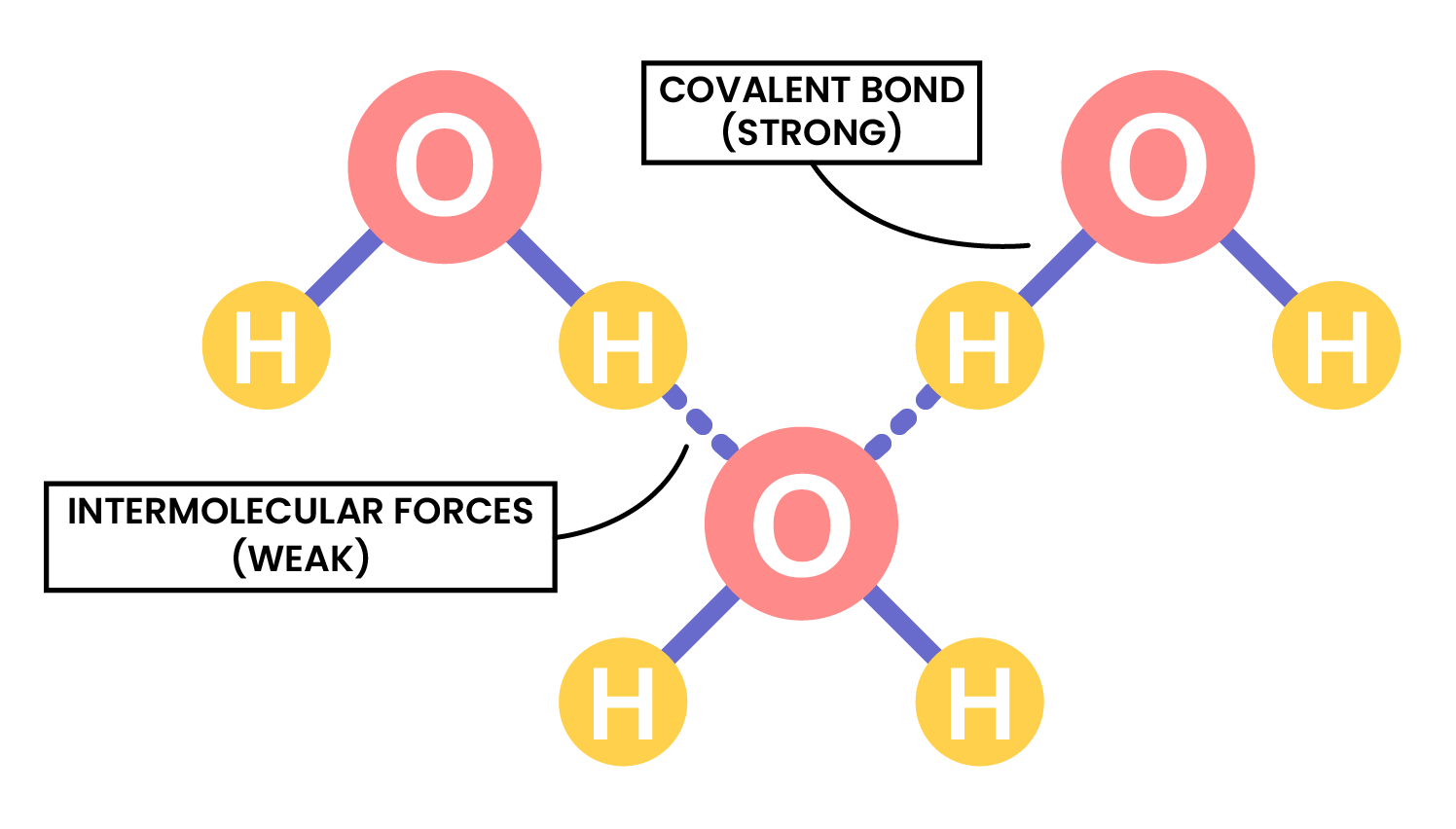
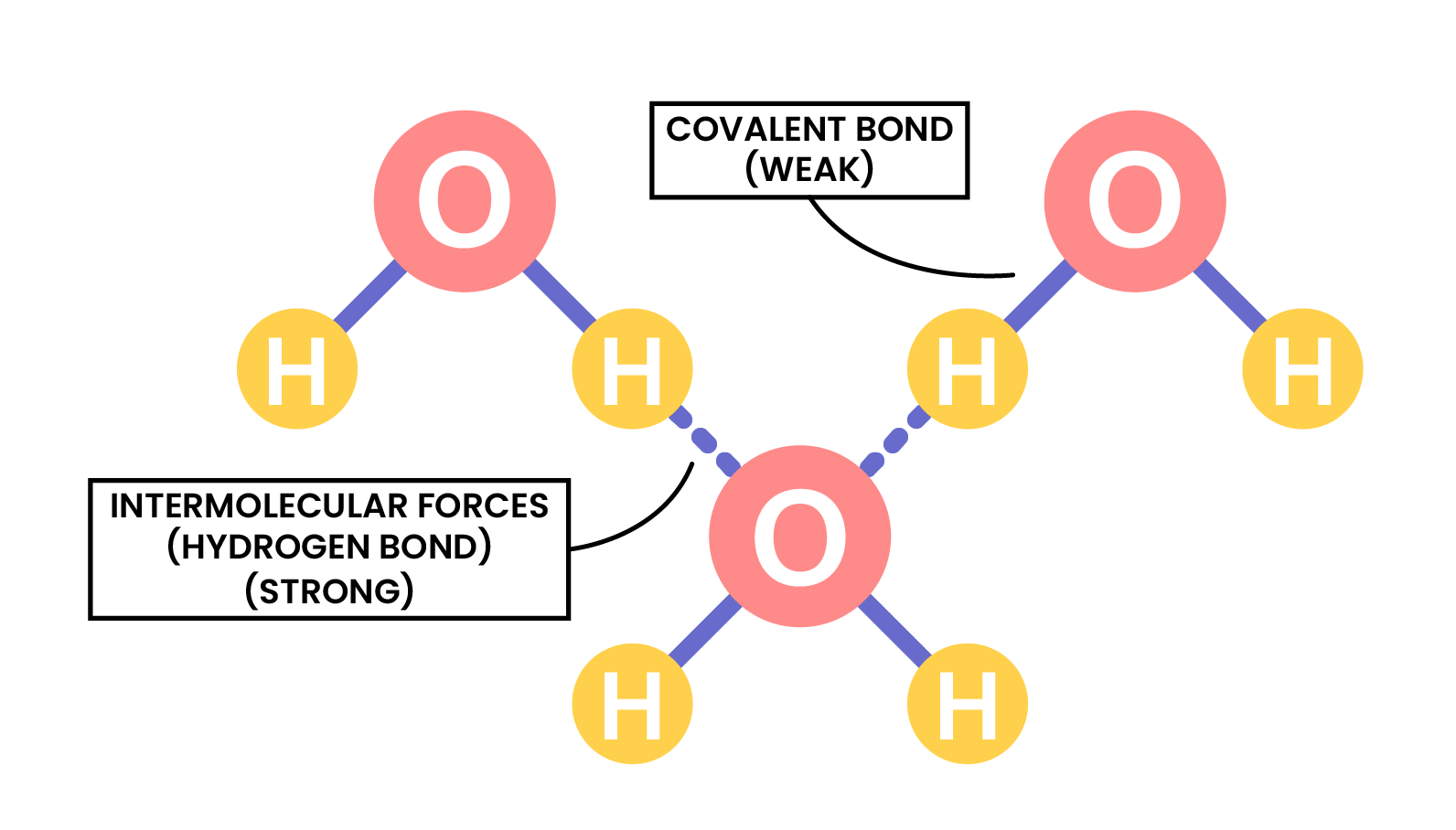
Two or more atoms covalently bonded together to form molecules - There are weak intermolecular forces between individual molecules
For example, in water molecules:
- Two hydrogen atoms and one oxygen atoms covalently bonded to form a water molecule
- There are weak intermolecular forces between the water molecules
In a molecule:
- Electrons shared are called bonding electrons
- Electrons on the outer shell that are not shared are called non-bonding electrons
- Electrons are all fixed (no free electrons), therefore simple covalent molecules
do not conduct electricity
1.7.2 Understand covalent bonds in terms of electrostatic attractions
COVALENT BONDING
Electrostatic attraction between the positive nuclei of the atoms and the shared pairs of negative electrons
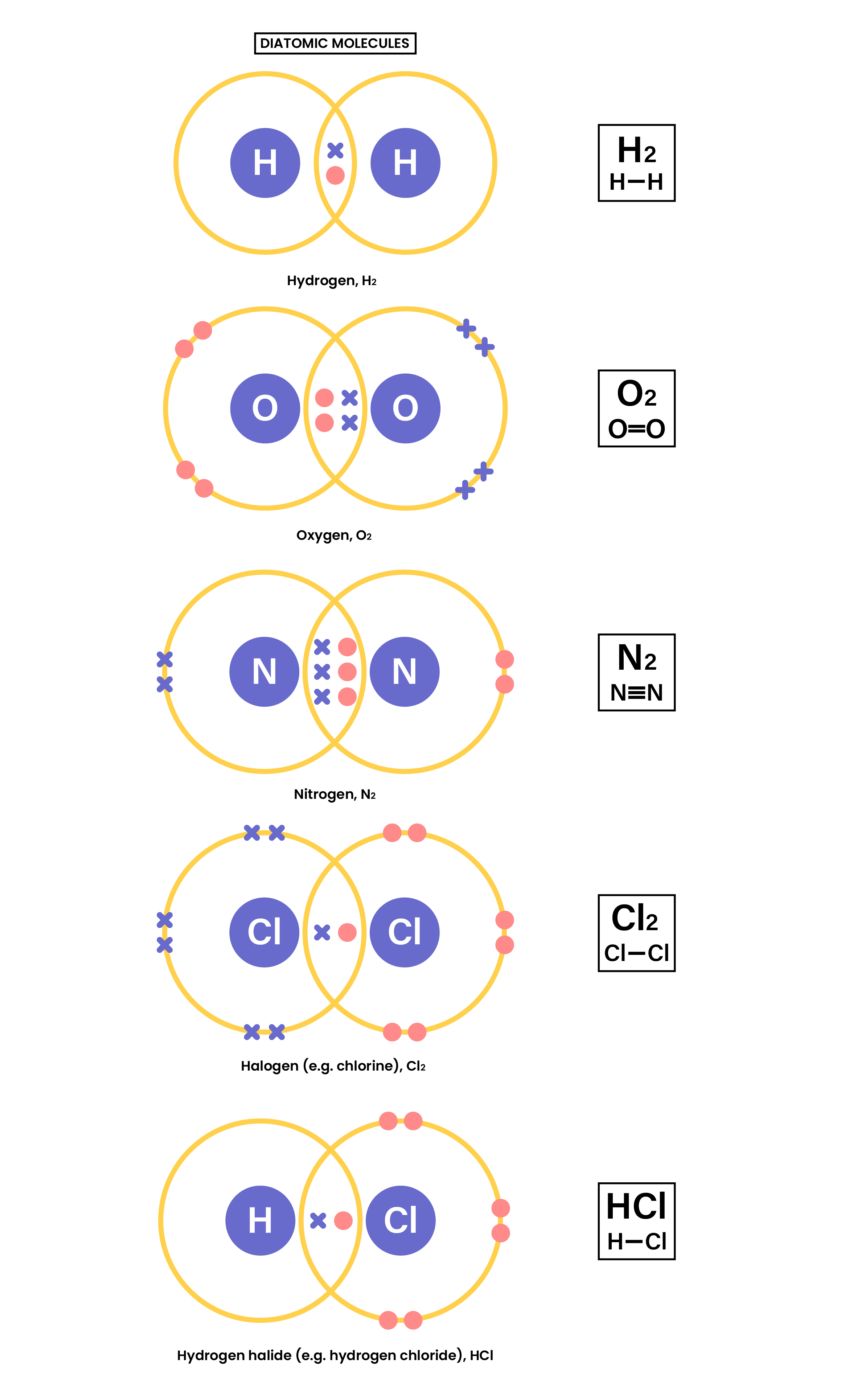
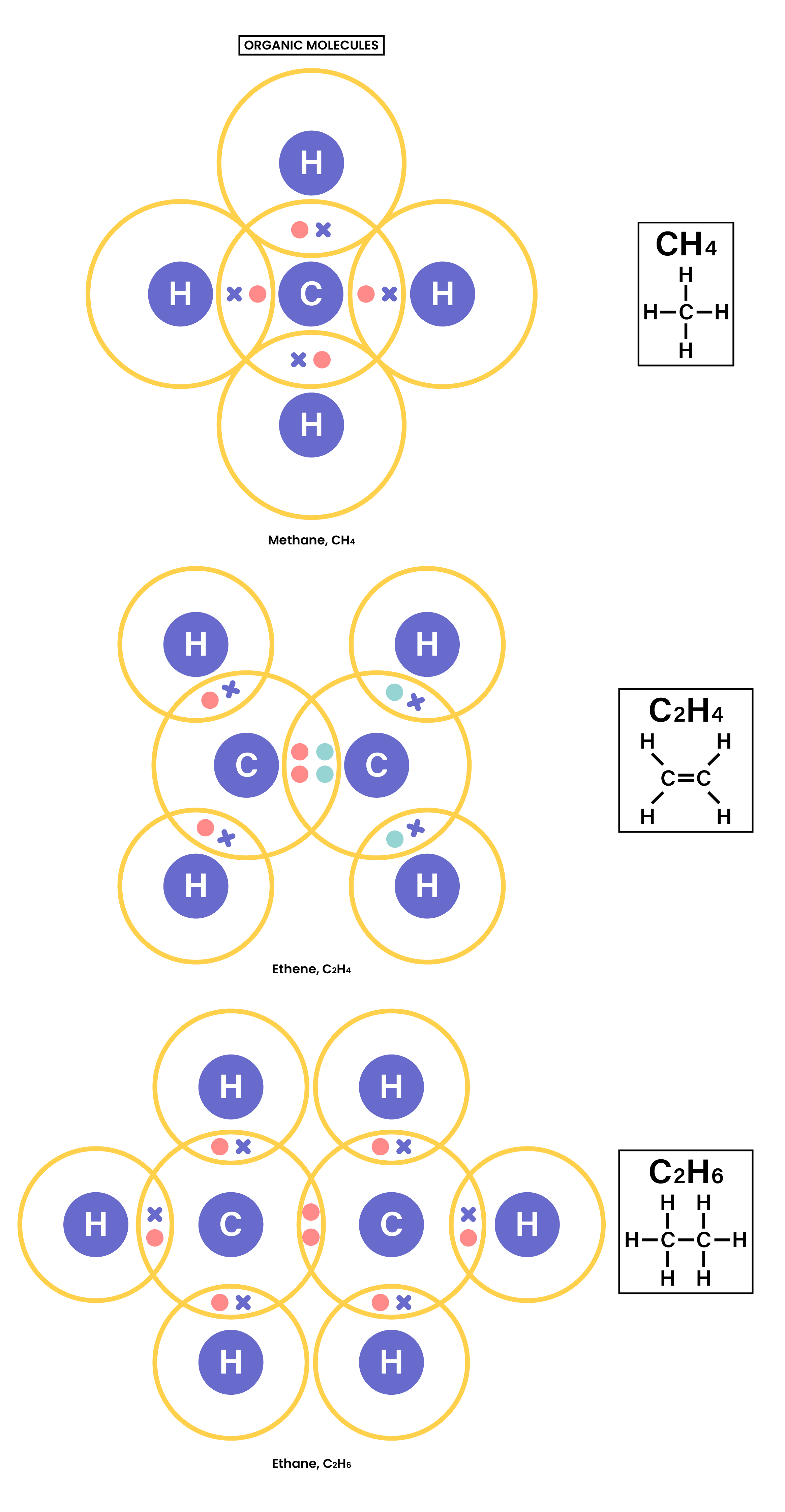
1.7.3 Understand how to use dot-and-cross diagrams to represent covalent bonds in:
- Diatomic molecules, including hydrogen, oxygen, nitrogen, halogens and hydrogen halides
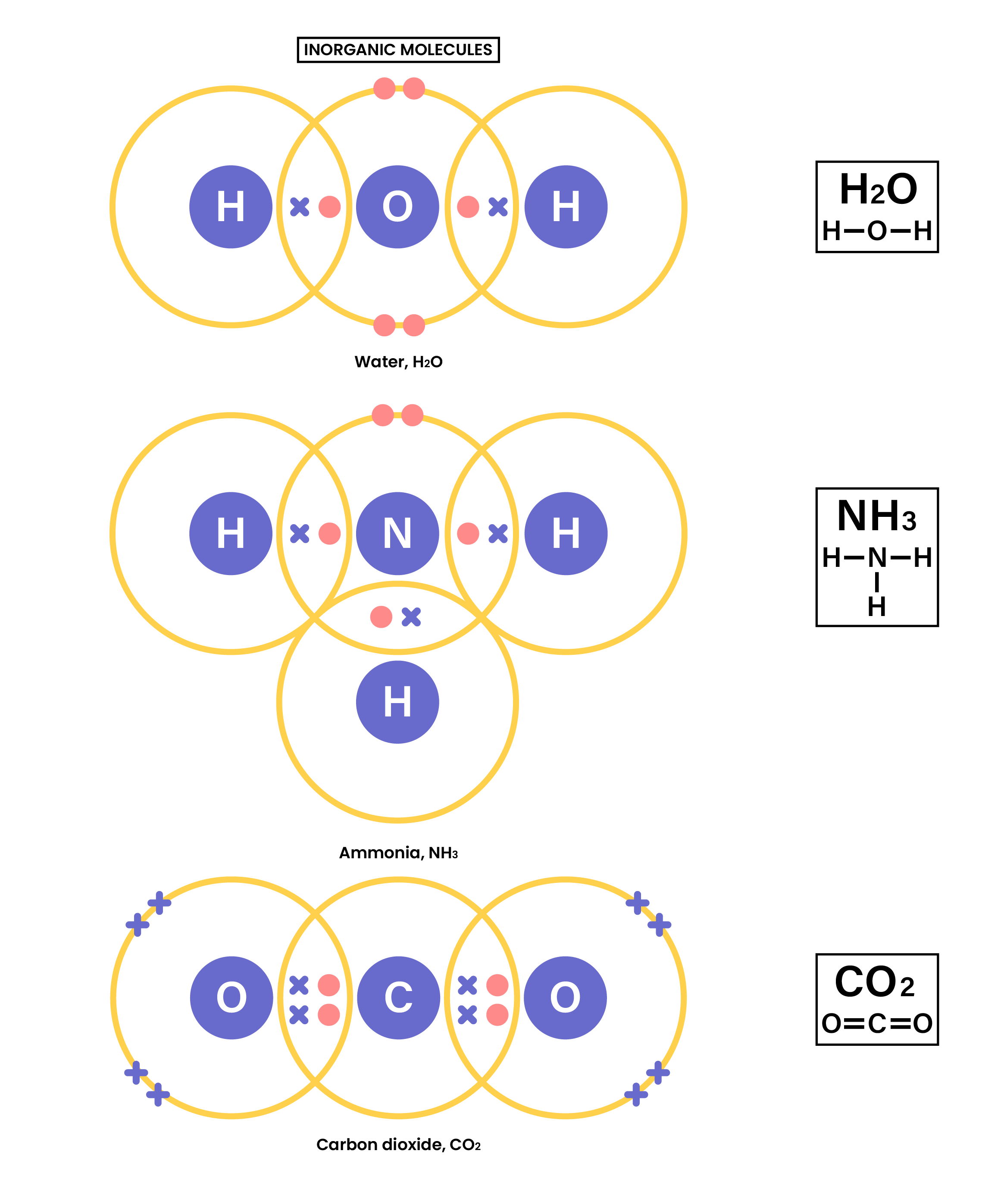
- Inorganic molecules including water, ammonia and carbon dioxide
- Organic molecules containing up to two carbon atoms, including methane, ethane, ethene and those containing halogen atoms.
1.7.4 Explain why substances with a simple molecular structures are gases or liquids, or solids with low melting and boiling points
the term intermolecular forces of attraction can be used to represent all forces between molecules
INTERMOLECULAR FORCES
- Covalent bonds are formed in between atoms in a molecule
- Intermolecular forces are formed between molecules
STATE OF MATTER
- The state of matter of substances depend on how strongly the particles are bonded together
- In simple molecular structures, the molecules are bonded together by weak intermolecular forces
- Therefore, it is easier to break the intermolecular forces between molecules (not covalent) in boiling or melting
- Thus, the boiling and melting point of simple molecules are lower
1.7.5 Explain why the melting and boiling points of substances with simple molecular structures increase, in general, with increasing relative molecular mass
INTERMOLECULAR FORCES INCREASE WITH THE SIZE OF THE MOLECULES
- Molecules with greater relative molecular masses are larger
- There are more electrons in the molecule to be polarised
- Therefore, there are stronger intermolecular forces between the molecules
- Larger molecules have higher melting and boiling points
1.7.6 Explain why substances with giant covalent structures are solids with high melting and boiling points
GIANT COVALENT STRUCTURES
- They have a huge number of non-metal atoms bonded to other non-metal atoms via strong covalent bonds
- These structures can also be called giant lattices
- They have a fixed ratio of atoms in the overall structure
- Three common macromolecules:
- Diamond
- Graphite
- C60 fullerene
1.7.7 Explain how the structures of diamond, graphite and C60 fullerene influence their physical properties, including electrical conductivity and hardness
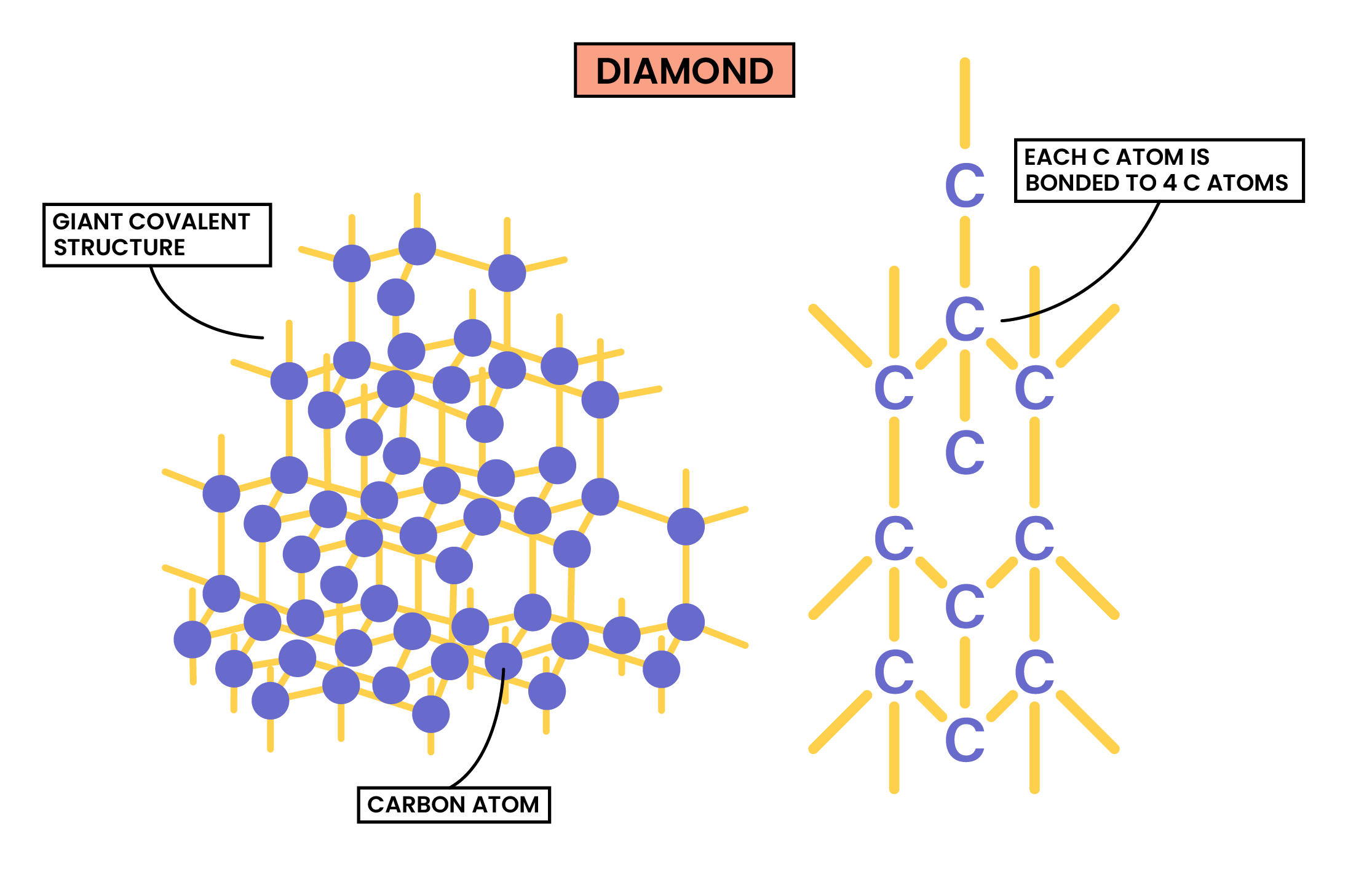
DIAMOND
- Diamond, graphite and C60 fullerene are allotropes of carbon
- Allotropes: each of two or more different physical forms in which an element can exist
- In diamond, each carbon atom bonds with four other carbons, forming a tetrahedron
- All covalent bonds are strong intramolecular forces
- There is no intermolecular force
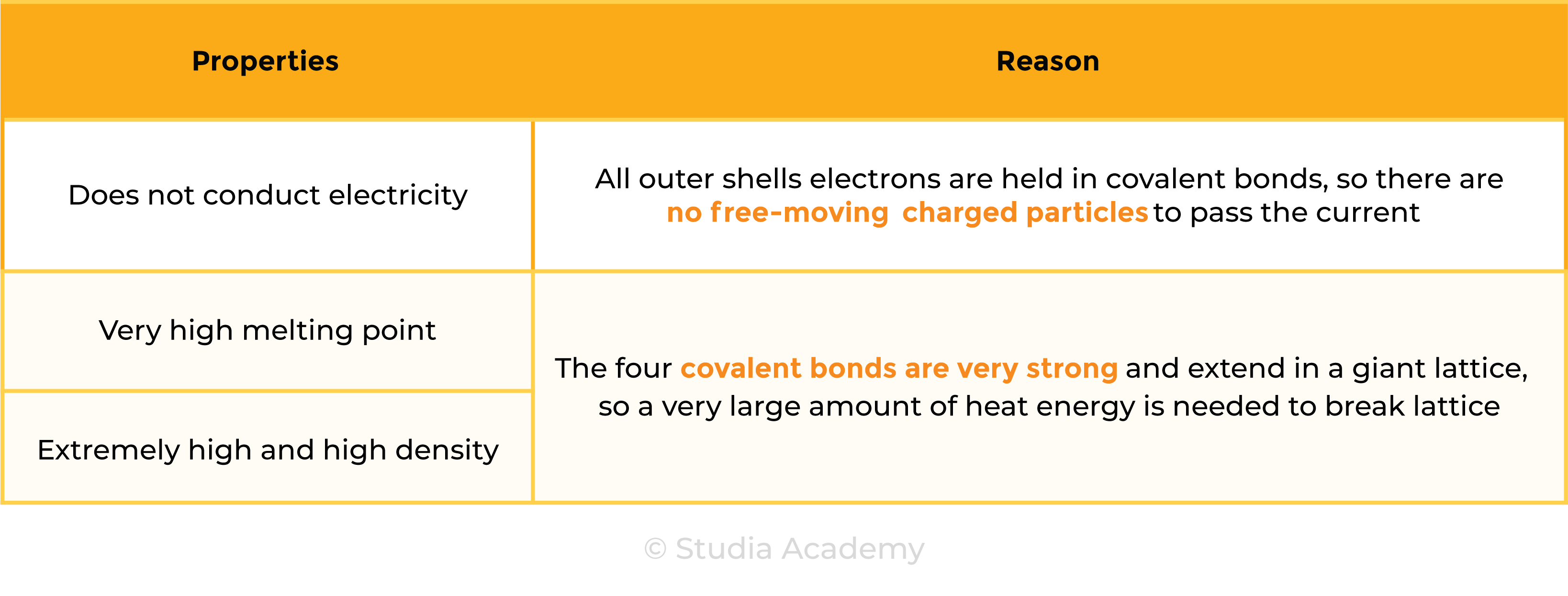
Properties of Diamond
USES OF DIAMOND
- Diamond is used in jewellery and for coating blades in cutting tools
- The cutting edges of discs used to cut bricks and concrete are tipped with diamonds
- Heavy-duty drill bits and tooling equipment are also diamond tipped
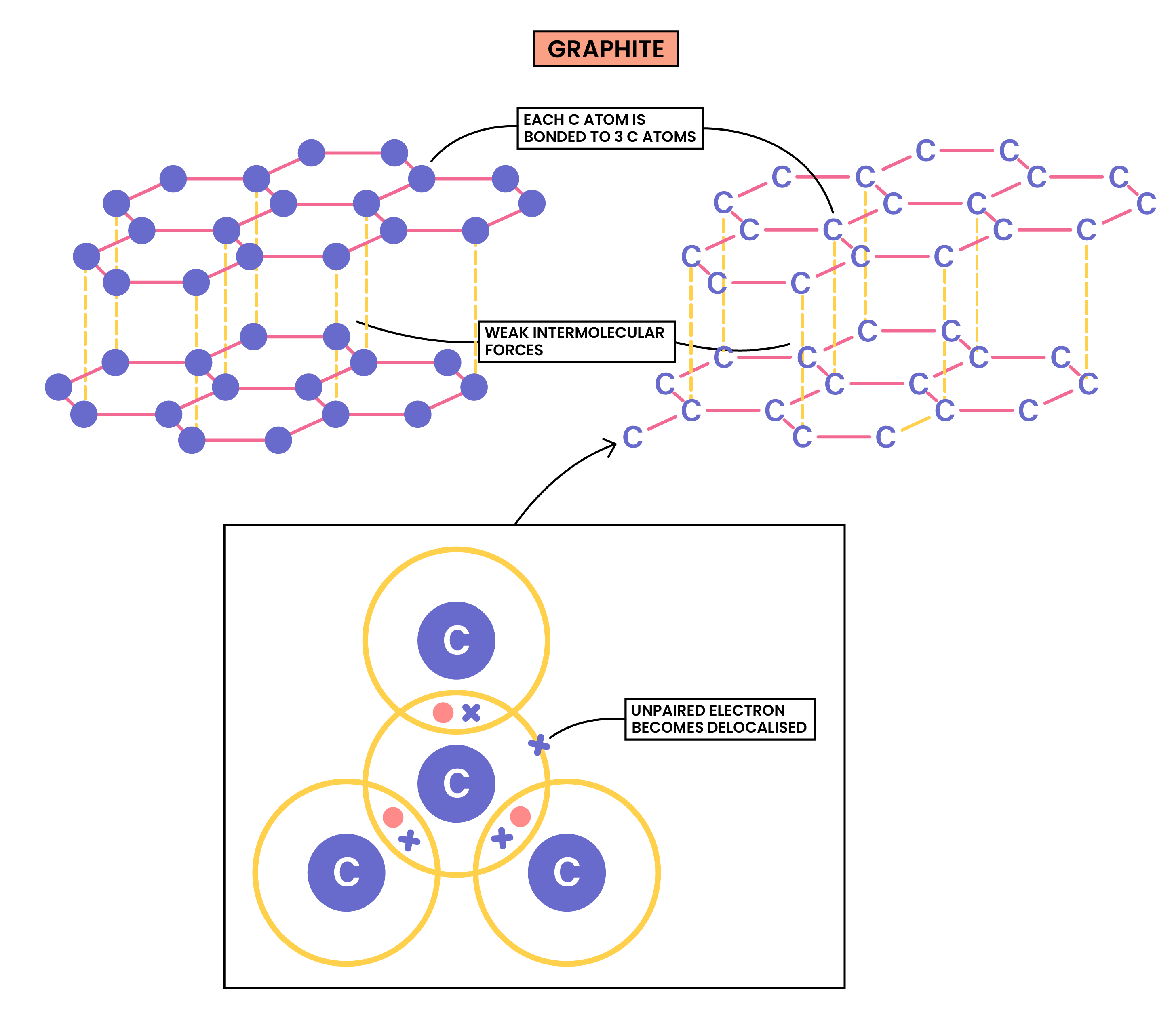
GRAPHITE
- Each carbon atom in graphite is bonded to three others
- Form layers of hexagons, leaving one free electron per carbon atom
- The free electrons move in between the layers
- There are strong intramolecular bonds between carbon atoms
- There are also weak intermolecular forces between the layers
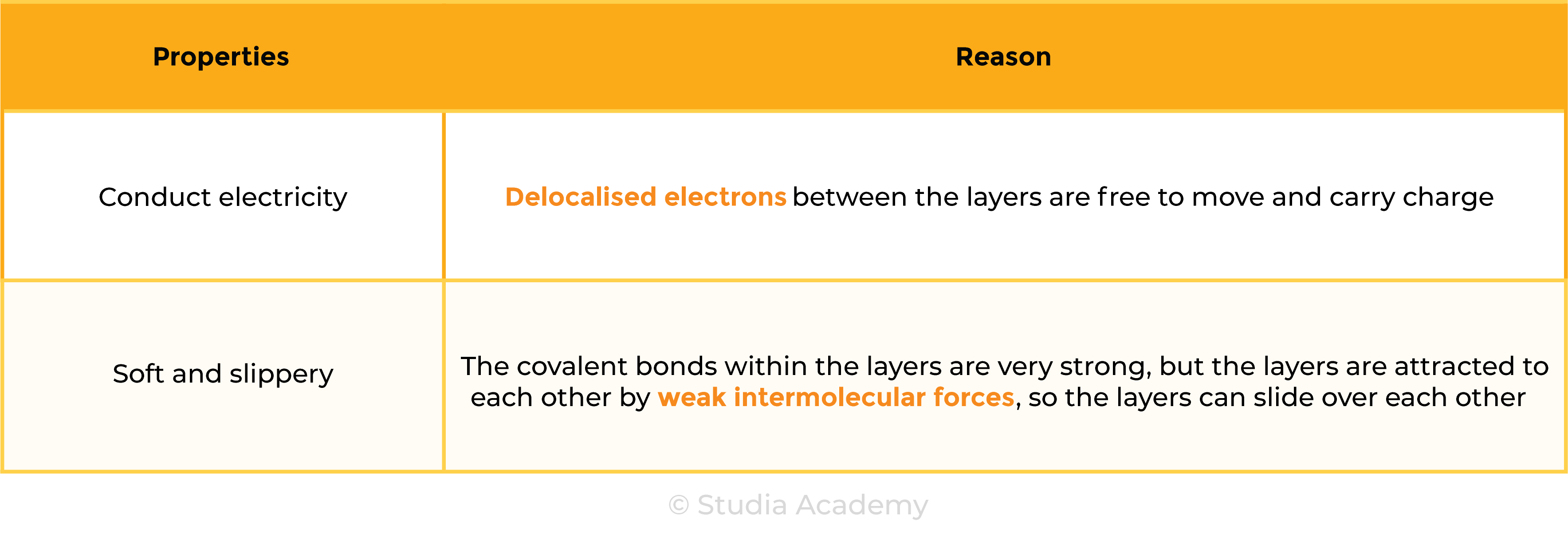
Properties of Graphite
USES OF GRAPHITE
- It is used in pencils
- It can be used as an industrial lubricant, in engines and in locks
- It is also used to make inert electrodes for electrolysis, which is particularly important in the extraction of metals such as aluminium
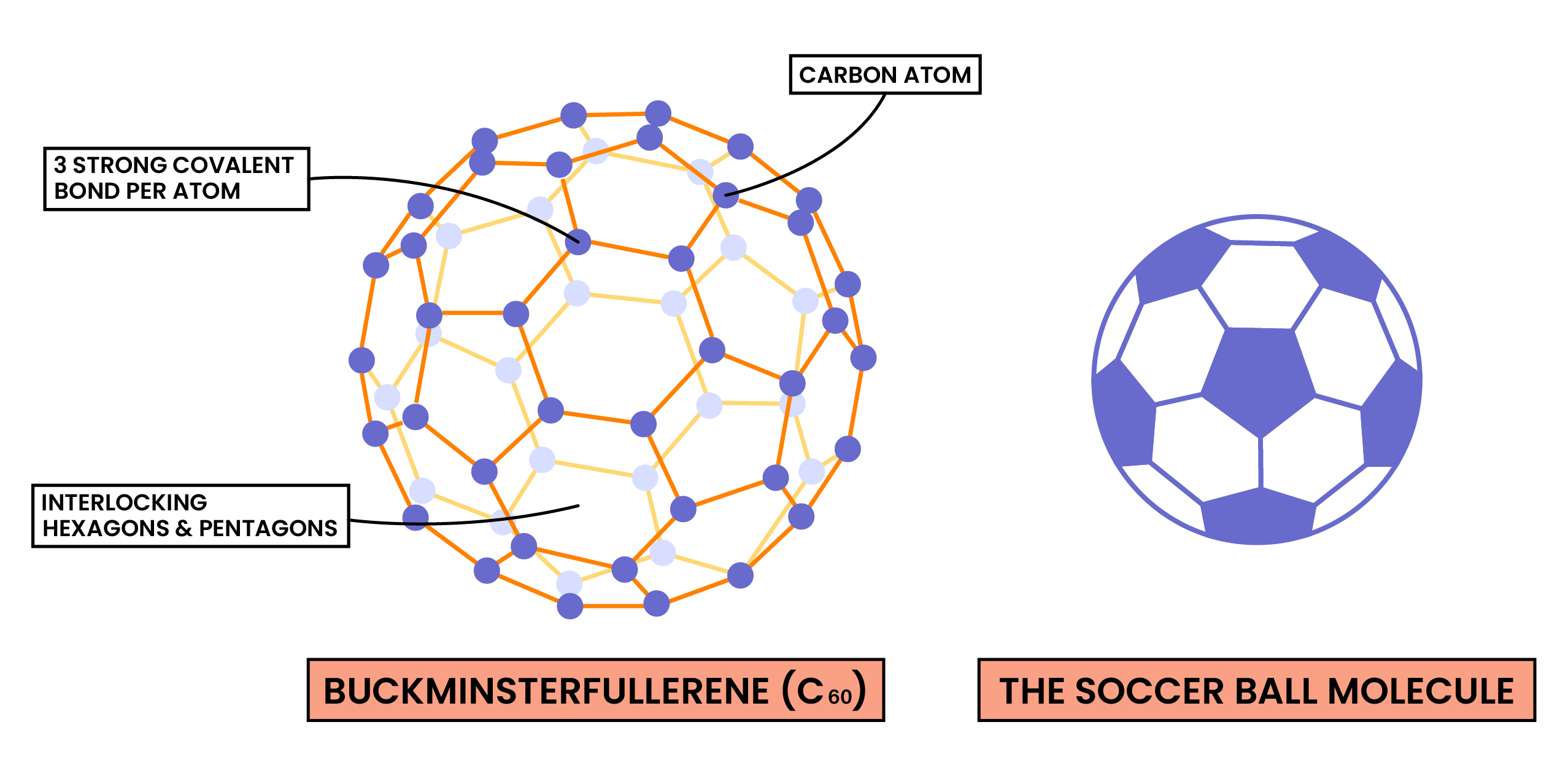
C60 Fullerene
- Fullerenes are a group of carbon allotropes in which carbon atoms form hollow tubes or spheres
- The first fullerene to be discovered was buckminsterfullerene which is also known as a “buckyball”
- In this fullerene, 60 carbon atoms are joined together forming 20 hexagons and 12 pentagons which produce a hollow sphere that is the exact shape of a soccer ball
Uses of C60 fullerene
- Fullerenes can be used to trap other molecules by forming around the target molecule and capturing it, making them useful for targeted drug delivery systems
- They also have a huge surface area and are useful for trapping catalyst molecules onto their surfaces making them easily accessible to reactants so catalysis can take place
- Some fullerenes are excellent lubricants and are starting to be used in many industrial processes
1.7.8 Know that covalent compounds do not usually conduct electricity
Covalent compounds do not conduct electricity because
- There are no free-moving electrons
Exception: graphite
- Graphite conducts electricity because there are free-moving electrons between the layers

