REVISION NOTES
1.4.1 Understand how elements are arranged in the Periodic Table:
Elements on the periodic table are arranged in order of increasing atomic number.
GROUPS
PERIODS
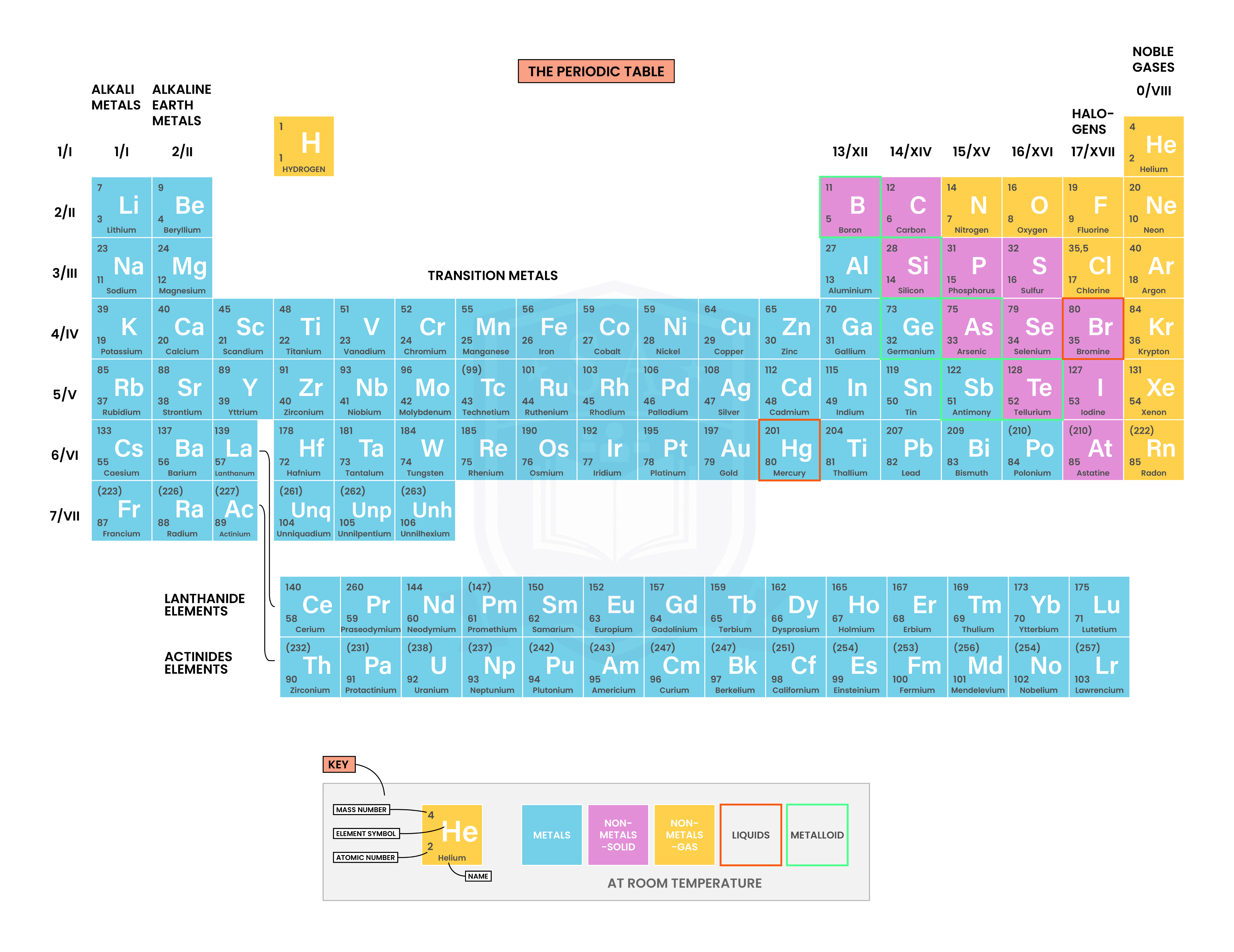
1.4.2 Understand how to deduce the electronic configurations of the first 20 elements from their positions in the Periodic Table
Deducing total number of electrons
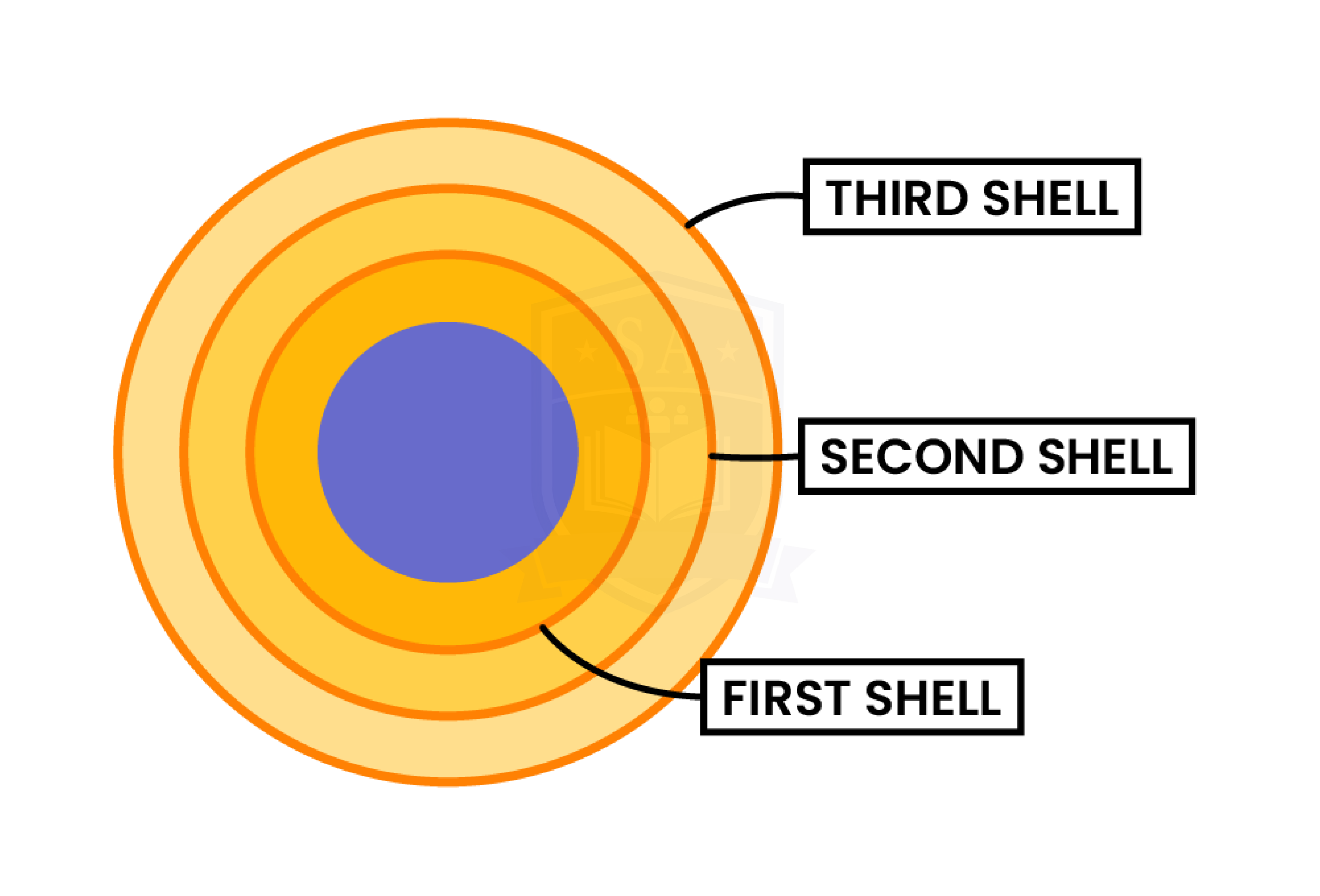
Each electron shell can accommodate a fixed number of electrons
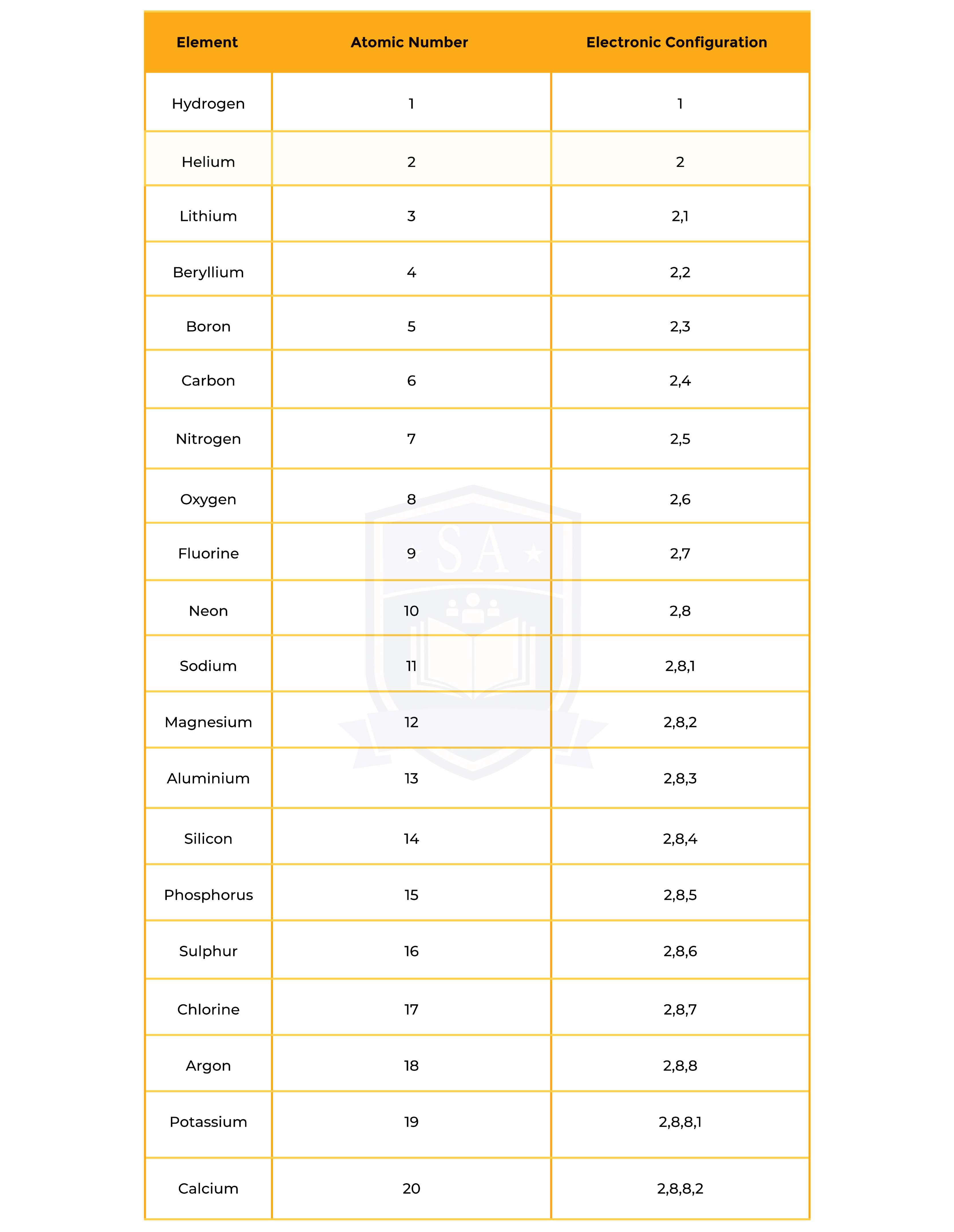
Electronic Configuration of First 20 Elements
1.4.3 Understand how to use electrical conductivity and the acid-base character of oxides to classify elements as metals or non-metals

1.4.4 Identify an element as a metal or a non-metal according to its position in the Periodic Table
In general,
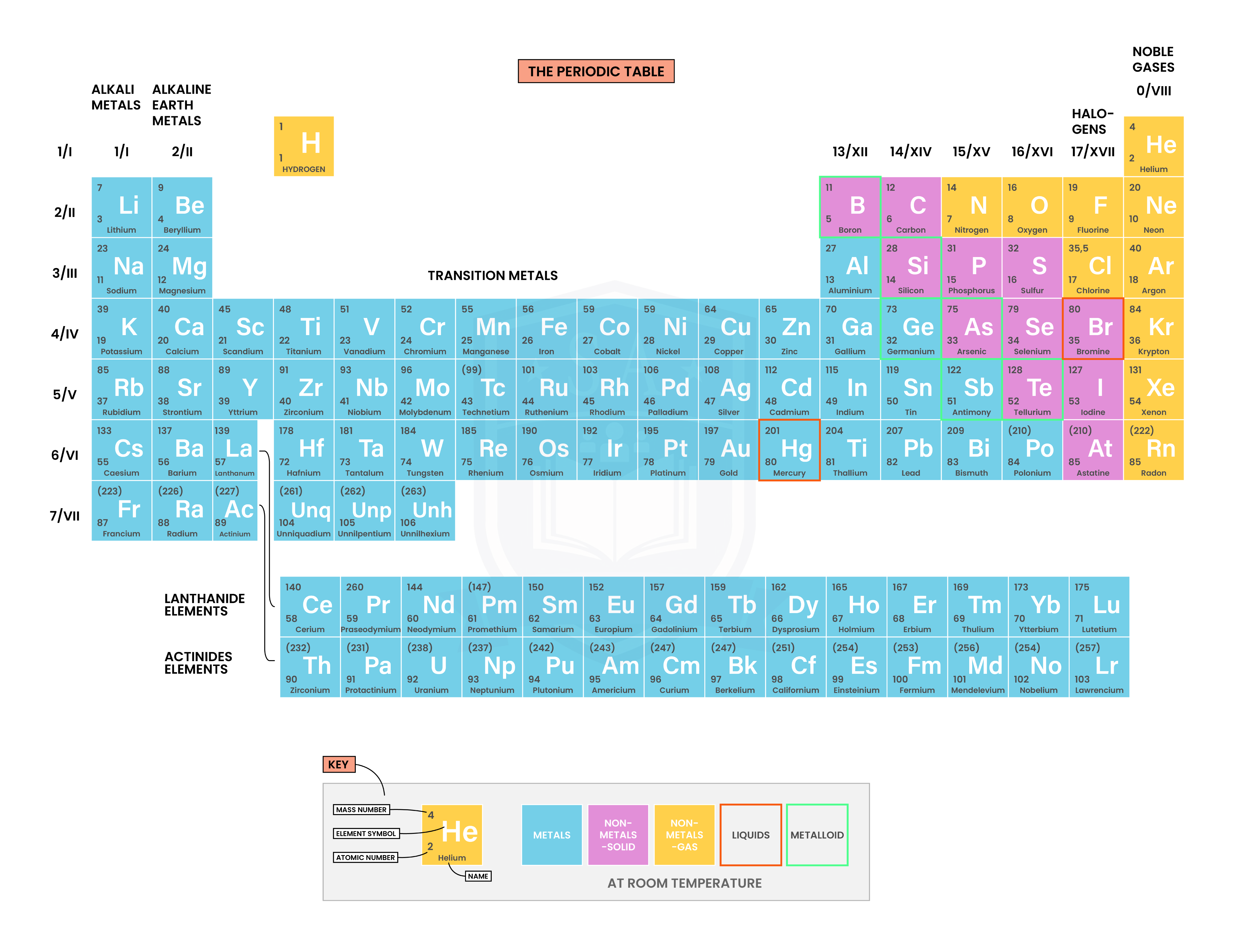
1.4.5 Understand how the electronic configuration of a main group element is related to its position in the Periodic Table
Period number: number of electron shells
Group number: number of electrons in the outer shell

1.4.6 Understand why elements in the same group of the Periodic Table have similar chemical properties
1.4.7 Understand why the noble gases (Group 0) do not readily react
© 2025 Studia Academy. All rights reserved.