REVISION NOTES
IGCSE Edexcel Chemistry
4.8 Synthetic Polymers
4.8.1 Know that an addition polymer is formed by joining up many small molecules called monomers
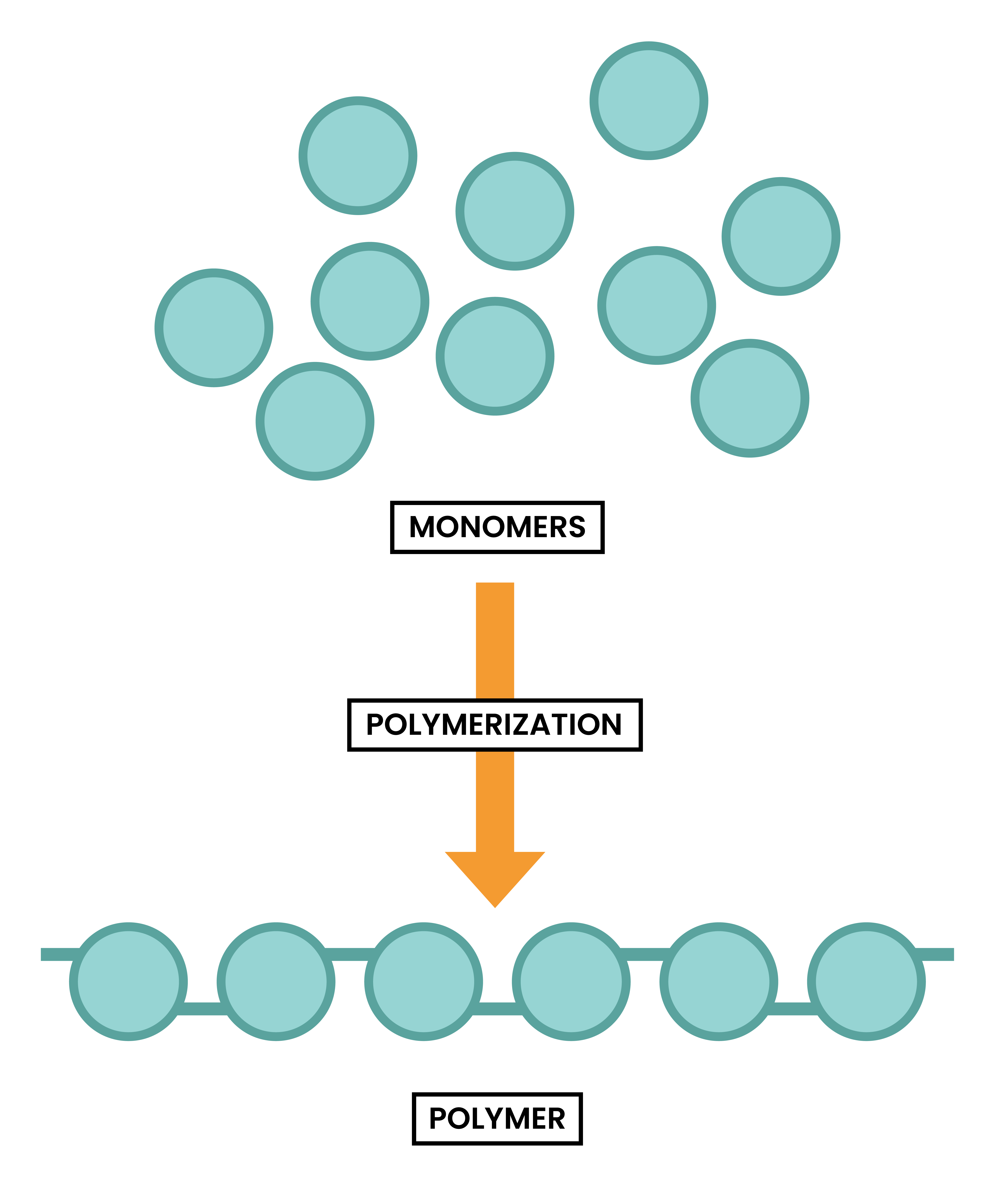
ADDITION POLYMERS
- Addition polymer is formed when many small molecules join together
- The process is called addition polymerisation
- The small molecules are called monomers (like lego building blocks)
4.8.2 Understand how to draw the repeat unit of an addition polymer, including poly(ethene), poly(propene), poly(chloroethene) and (poly)tetrafluoroethene
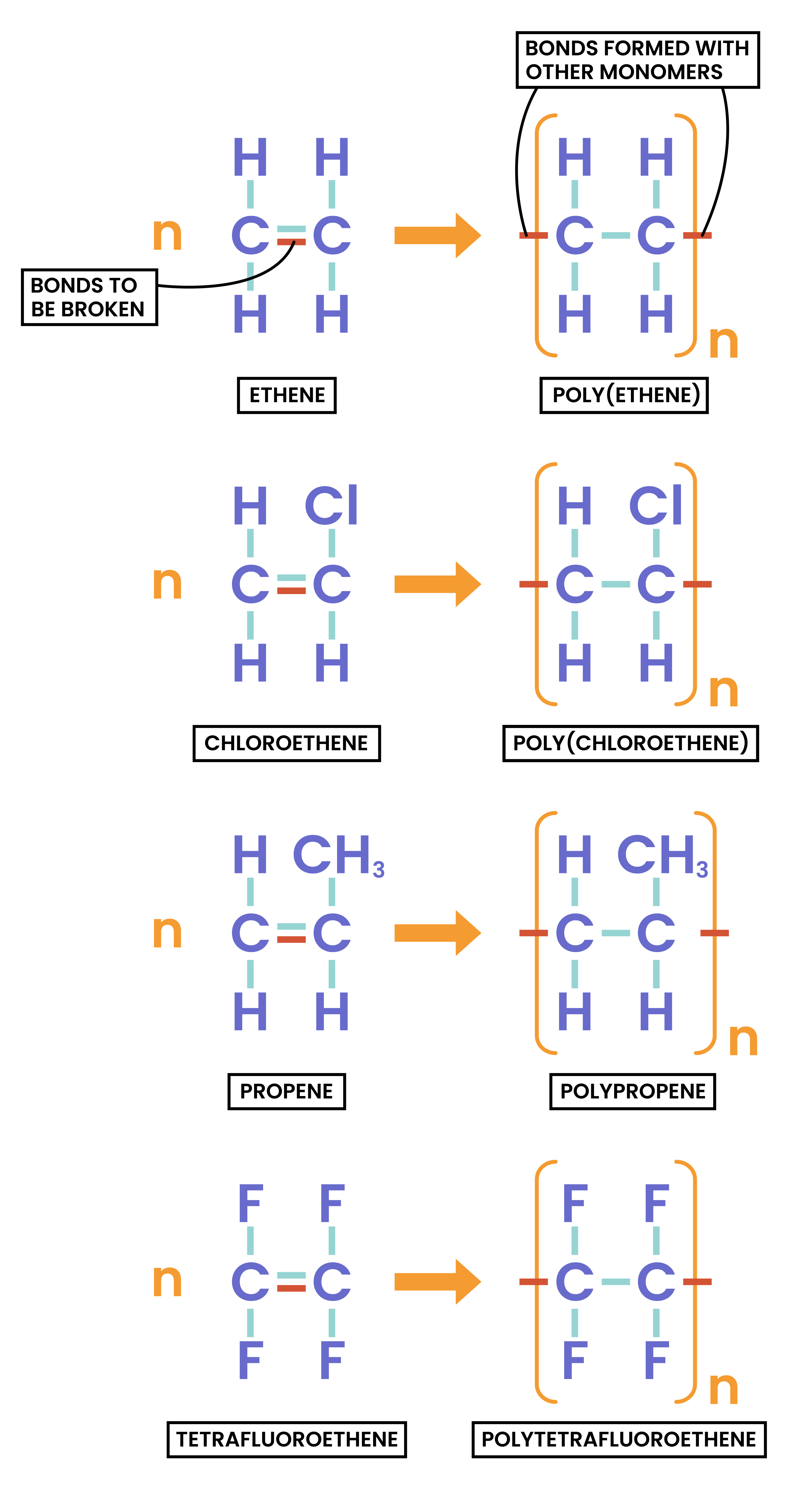
FORMATION OF POLYMERS
- Polymers are formed by joining many monomers with C=C bonds together
- One of the bonds in each C=C bonds breaks and forms a bond with the adjacent monomer (like holding hands together)
- Monomers can be a variety of compounds with C=C bonds
- E.g. ethene, propene, chloroethene and tetrafluoroethene
NAMING POLYMERS
- Prefix: poly–
- + name of the monomer
- E.g. propene → poly(propene)
4.8.3 Understand how to deduce the structure of a monomer from the repeat unit of an addition polymer and vice versa
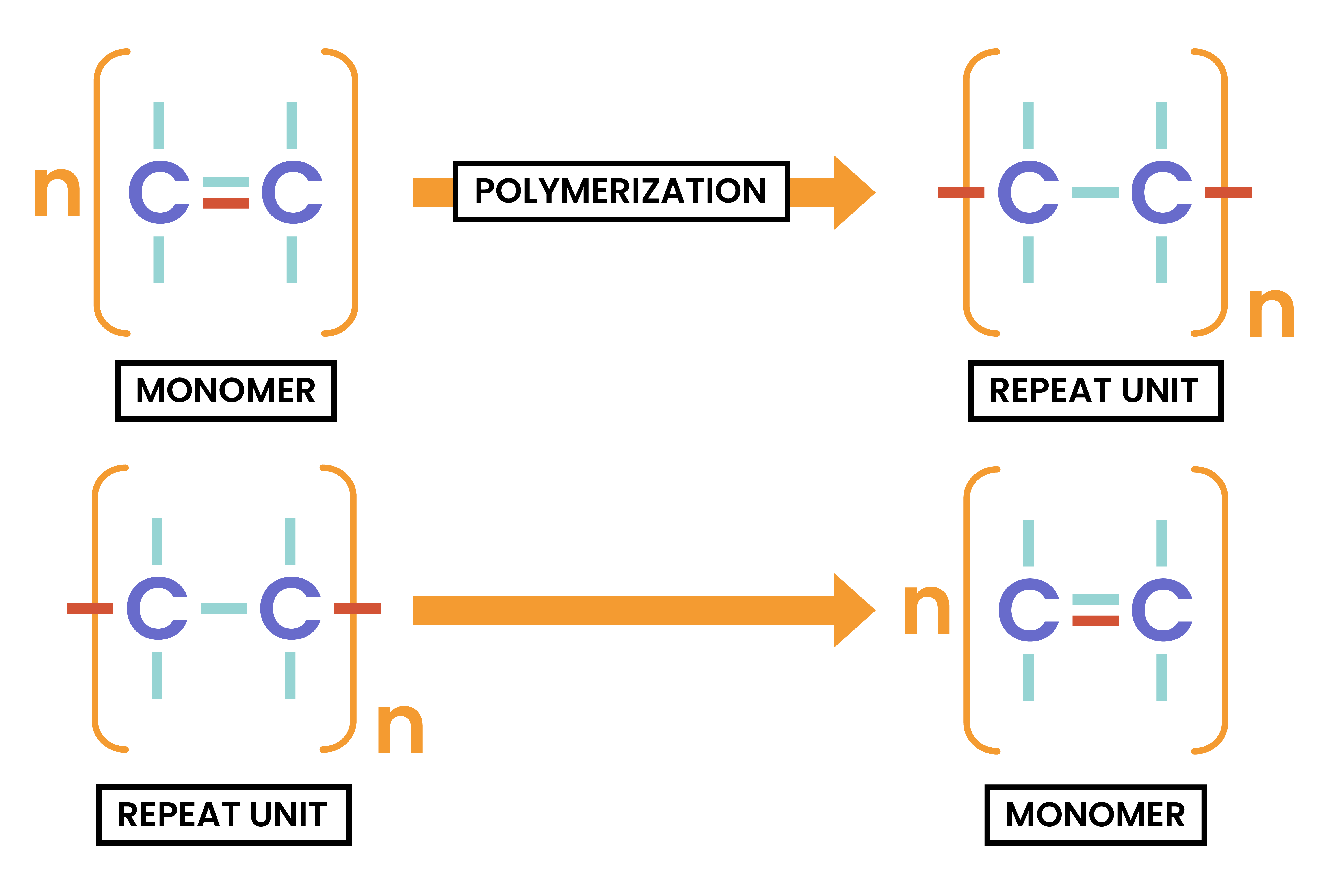
DEDUCING POLYMER REPEAT UNIT FROM MONOMER
- Draw several monomers next to each other
- Remove one of the bonds in C=C
- New bonds are formed in between the monomers, connecting monomers together
DEDUCING MONOMER FROM POLYMER UNIT
- Find the repeating unit in the polymer
- Draw only one repeating unit
- Remove the connecting bonds
- Add a second bond between C and C to form C=C bond
4.8.4 Explain problems in the disposal of addition polymers, including:
- Their inertness and inability to biodegrade
- The production of toxic gases when they are burned.
PROBLEMS IN DISPOSAL OF ADDITION POLYMERS
1. Landfill
- Addition polymers are formed by joining many monomers together with strong C-C bonds
- As a result, addition polymers are unreactive and chemically inert
- Polymers do not easily biodegrade
2. Incineration
- If polymers are burned, toxic gases may be produced
- Complete combustion: carbon dioxide (greenhouse gas)
- Incomplete combustion: carbon monoxide (toxic gas that reduces the capacity of blood to carry oxygen)
- Burning polymers with chlorine (e.g. PVC): hydrogen chloride gas (toxic gas)
4.8.5C Know that condensation polymerisation, in which a dicarboxylic acid reacts with a diol, produces a polyester and water
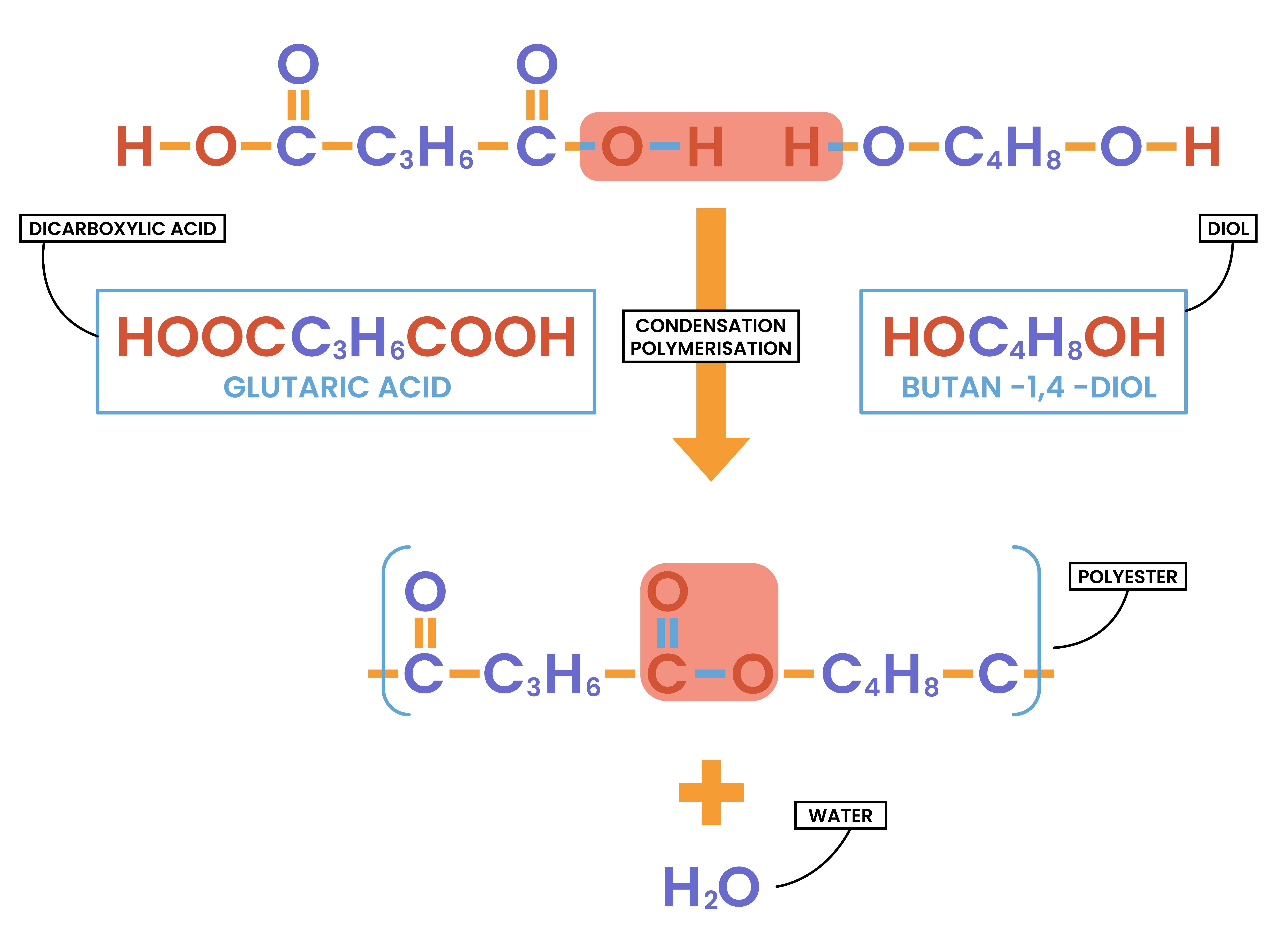
CONDENSATION POLYMERS
- Recall: alcohol + carboxylic acid → ester + H2O
- Dicarboxylic acid: functional group -COOH is present on both ends of the compound
- Diol: functional group -OH is present on both ends of the compound
- Condensation (esterification) can occur on both ends of alcohol and carboxylic acid compounds, forming a long polymer
4.8.6C Understand how to write the structural and displayed formula of a polyester, showing the repeat unit, given the formulae of the monomers from which it is formed including the reaction of ethanedioic acid and ethanediol:
- There should be several ester functional groups (-COO-) present in the polymer as repeating units
4.8.7C Know that some polyesters, known as biopolyesters, are biodegradable
BIOPOLYESTERS
- Polyesters that are biodegradable are called biopolyesters

