REVISION NOTES
IGCSE Edexcel Chemistry
4.2 Crude Oil
4.2.1 Know that crude oil is a mixture of hydrocarbons
CRUDE OIL
- Mixture of hydrocarbons
- Contains molecules in which carbon atoms are in chains or in rings
- Important source of useful substances
- Finite source
4.2.2 Describe how the industrial process of fractional distillation separates crude oil into fractions
FRACTIONAL DISTILLATION
- Crude oil is separated by fractional distillation
- Fractional distillation is based on different boiling points of hydrocarbons
- The longer the carbon chain, the higher the boiling point
PROCESS IN SEPARATING CRUE OILS TO FRACTIONS
- Crude oil is heated
- Vaporised oil rises up the fractionating column
- Fractionating column is hotter at the bottom than at top
- Shorter hydrocarbons have lower boiling point and rise towards the top
4.2.3 Know the names and uses of the main fractions obtained from crude oil: refinery gases, gasoline, kerosene, diesel, fuel oil and bitumen
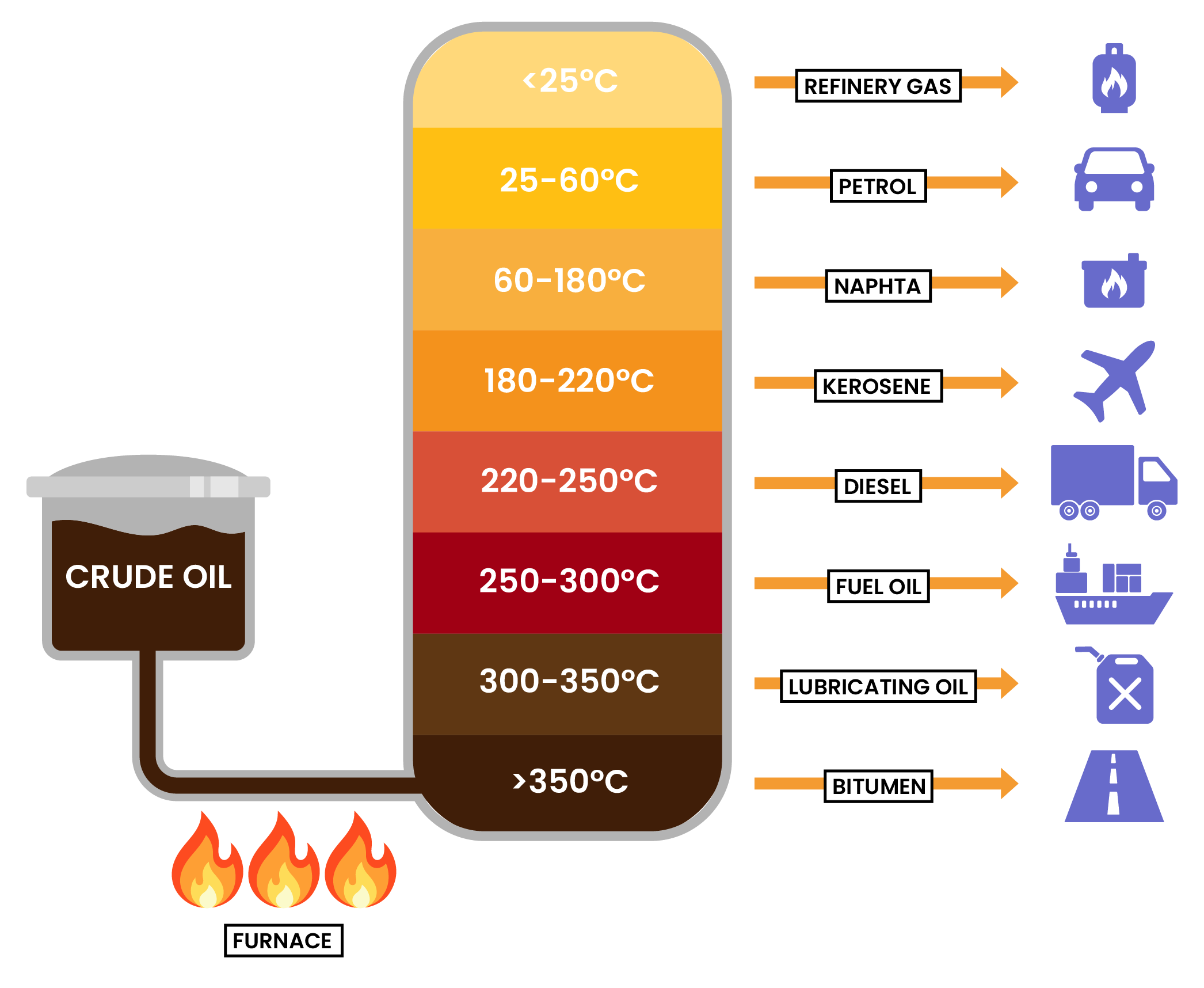
4.2.4 Know the trend in colour, boiling point and viscosity of the main fractions
- The sizes of hydrocarbon molecules affect some of their properties
- If a molecule is longer, then:
- Higher boiling point
- Higher temperature at which the fraction vaporises or condenses
- More viscous (less runny)
- Darker in colour
- This is due to stronger intermolecular forces attraction between larger molecules
4.2.5 Know that a fuel is a substance that, when burned, releases heat energy
FUEL
A substance that releases energy as heat when burned
4.2.6 Know the possible products of complete and incomplete combustion of hydrocarbons with oxygen in the air
COMPLETE COMBUSTION OF HYDROCARBONS
hydrocarbon + oxygen [sufficient] → carbon dioxide + water
INCOMPLETE COMBUSTION OF HYDROCARBONS
hydrocarbon + oxygen [insufficient] → carbon / carbon monoxide + water
4.2.7 understand why carbon monoxide is poisonous, in terms of its effect on the capacity of blood to transport oxygen
references to haemoglobin are not required
CARBON MONOXIDE
- Red blood cells in the blood carry oxygen around the body
- When carbon monoxide and oxygen are present at the same time, red blood cells tend to be more attracted to carbon monoxide
- Carbon monoxide thus prevents red blood cells from carrying oxygen around the body
4.2.8 Know that, in car engines, the temperature reached is high enough to allow nitrogen and oxygen from air to react, forming oxides of nitrogen
FORMATION OF OXIDES OF NITROGEN [TITLE]
- Temperature in car engines can be very high
- This allows nitrogen (N2) and oxygen (O2) from the air to react
- It forms oxides of nitrogen, for example:
- Nitrogen monoxide NO
- Nitrogen dioxide NO2
nitrogen + oxygen → nitrogen monoxide / nitrogen dioxide
N2 + O2 → NO / NO2
4.2.9 Explain how the combustion of some impurities in hydrocarbon fuels results in the formation of sulfur dioxide
FORMATION OF SULPHUR DIOXIDE
- Besides carbon and/or hydrogen, most fuels might contain sulphur as impurities
- Therefore, when fuels are burned, sulphur can be oxidised to produce sulphur dioxide SO2
sulphur + oxygen → sulphur dioxide
S + O2 → SO2
4.2.10 Understand how sulfur dioxide and oxides of nitrogen oxides contribute to acid rain
When sulphur dioxide and oxides of nitrogen are emitted into the atmosphere, they react with water (rainwater) to form acids
2SO2 + 2H2O + O2 → 2H2SO4
4NO2 + 2H2O + O2 → 4HNO3
- This makes rainwater even more acidic
- When acid rain falls, it:
- Alter the pH in soil and rivers, which then affects the ecosystem
- Corrodes limestone, which damages buildings and statues
4.2.11 Describe how long-chain alkanes are converted to alkenes and shorter-chain alkanes by catalytic cracking (using silica or alumina as the catalyst and a temperature in the range of 600–700 C)
LONG-CHAIN ALKANES
- Crude oils contain a larger proportion of long-chain alkanes
- They are less useful compared to short-chain alkanes
- Therefore, it is more beneficial and profitable to convert long-chain alkanes to short-chain alkanes through the process of catalytic cracking
CATALYTIC CRACKING
- The conversion of a longer chain alkane into a shorter chain alkane and alkenes
- It involves heating the hydrocarbons to vaporise them (600-700oC)
- Silica or alumina is used as catalyst (to speed up the process)
4.2.12 explain why cracking is necessary, in terms of the balance between supply and demand for different fractions
- Demand for shorter-chain alkanes is greater than that for longer-chain alkanes
- However, supply for longer-chain alkanes is greater, therefore, cracking is required to produce smaller-chain alkanes from longer-chain alkanes
- Shorter chain alkanes burn more cleanly
- Cracking produces alkenes which are used to make polymers

