REVISION NOTES
IGCSE Edexcel Chemistry
2.7 Acids, Bases and Salt Preparations
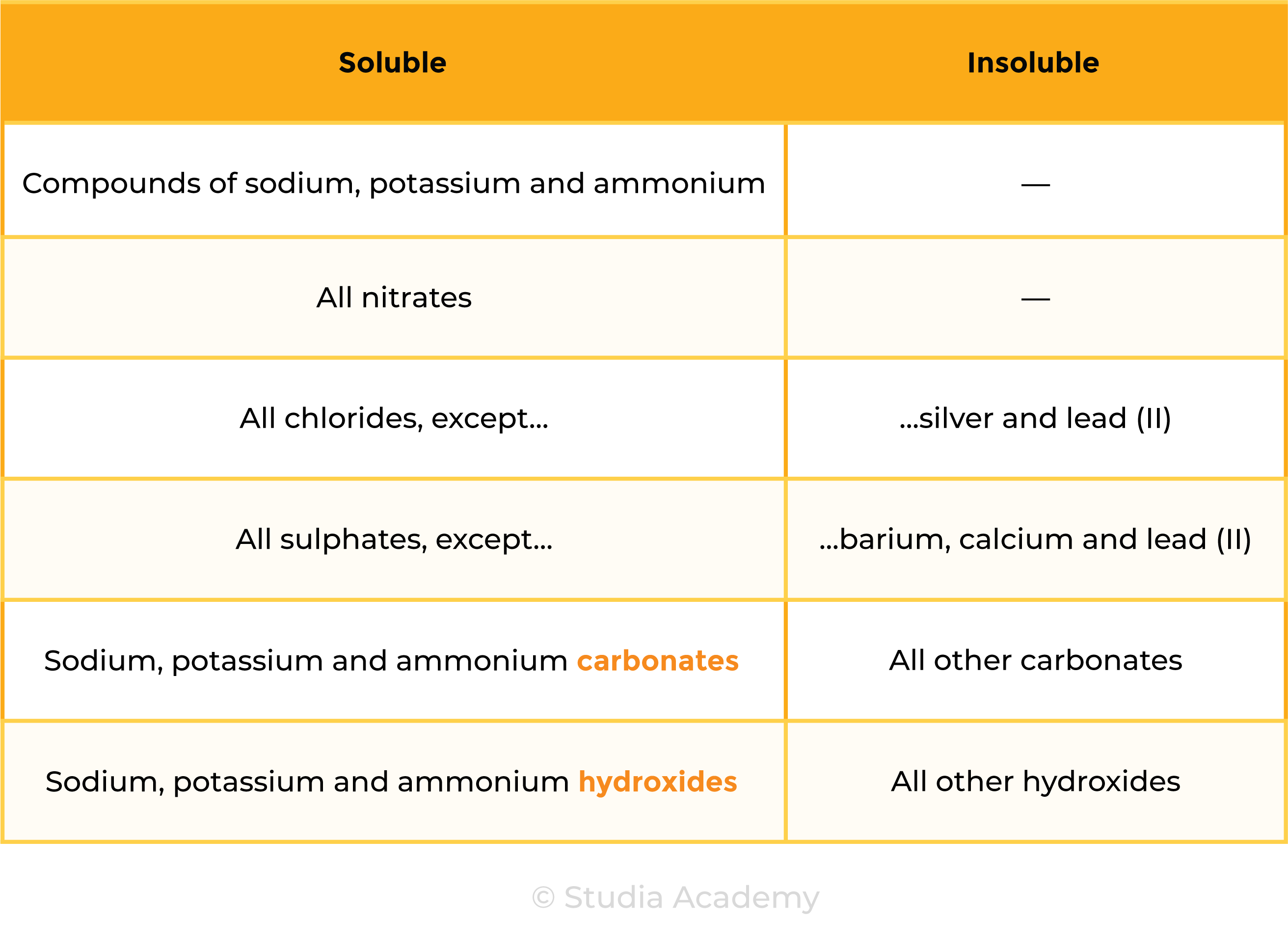
2.7.1 Know the general rules for predicting the solubility of ionic compounds in water:
- Common sodium, potassium and ammonium compounds are soluble
- All nitrates are soluble
- Common chlorides are soluble, except those of silver and lead(II)
- Common sulfates are soluble, except for those of barium, calcium and lead(II)
- Common carbonates are insoluble, except for those of sodium, potassium and ammonium
- Common hydroxides are insoluble except for those of sodium, potassium and calcium (calcium hydroxide is slightly soluble).
SOLUBILITY OF SALTS
- Ionic compounds are generally soluble in water, but there are exceptions
- Knowing the solubility of ionic compounds helps to determine the most appropriate method for salt preparation
2.7.2 Understand acids and bases in terms of proton transfer
- Earlier definition of acid and base can be extended in terms of proton transfer
- Acids: proton donors
- Bases: proton acceptors
- When a neutralisation reaction occurs, a proton (hydrogen ion) is transferred from an acid to a base
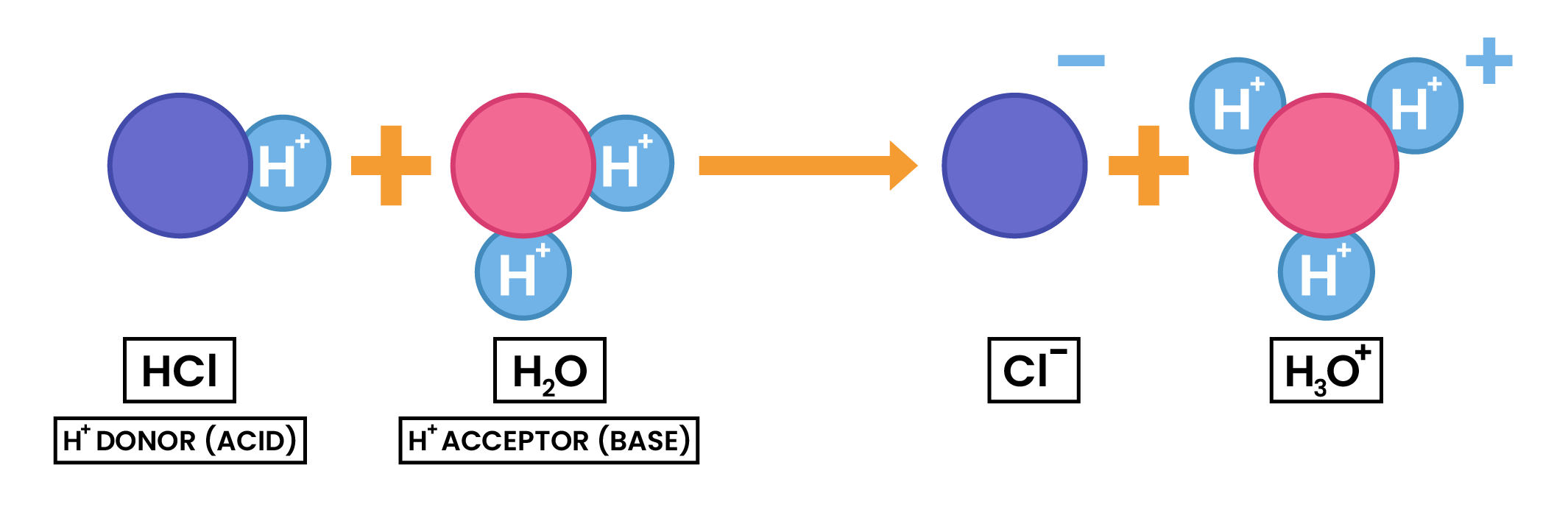
2.7.3 Understand that an acid is a proton donor and a base is a proton acceptor
Refer to 2.7.2
2.7.4 Describe the reactions of hydrochloric acid, sulfuric acid and nitric acid with metals, bases and metal carbonates (excluding the reactions between nitric acid and metals) to form salts
Reaction 1 Acid + Metal
Acid + Metal → Salt + Hydrogen
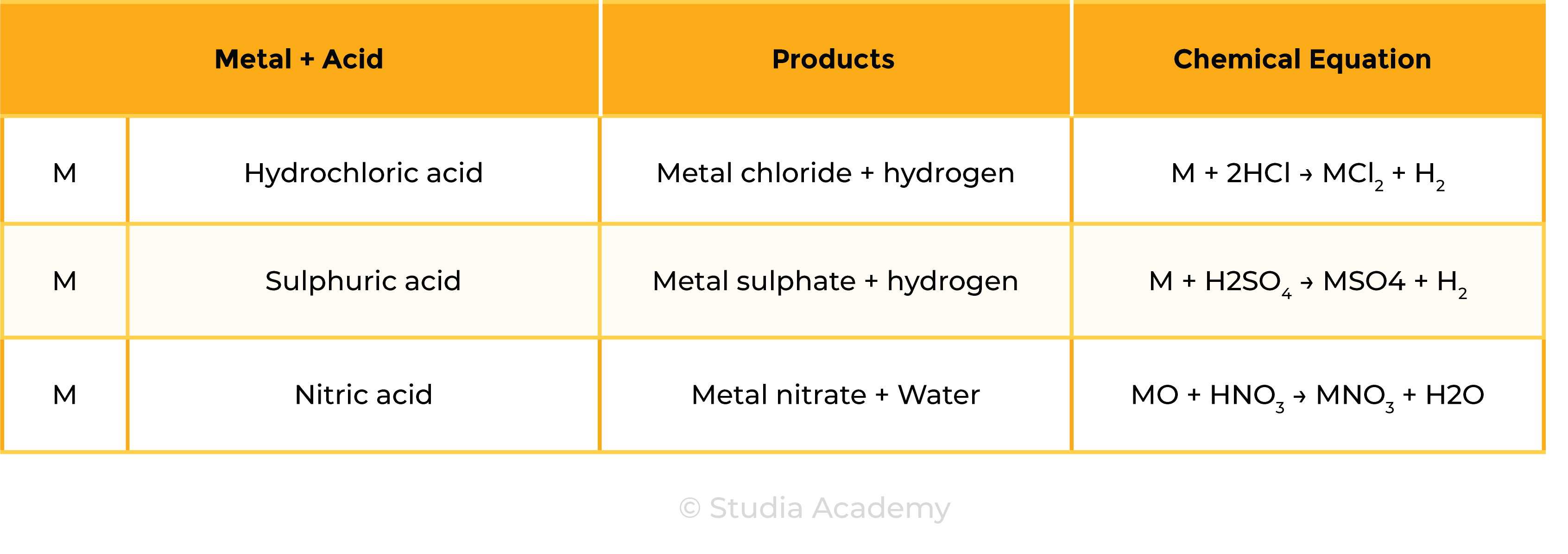
Examples
- Mg + H2SO4 → MgSO4 + H2
- Mg + 2HCl → MgCl2 + H2
Examples
- 2HCl + CuO → CuCl2 + H2O
- H2SO4 + 2NaOH → Na2SO4 + 2H2O
- HNO3 + KOH → KNO3 + H2O
Reaction 3 Acid + Metal Carbonate
Acid + Metal carbonate → Salt + Carbon dioxide + Water
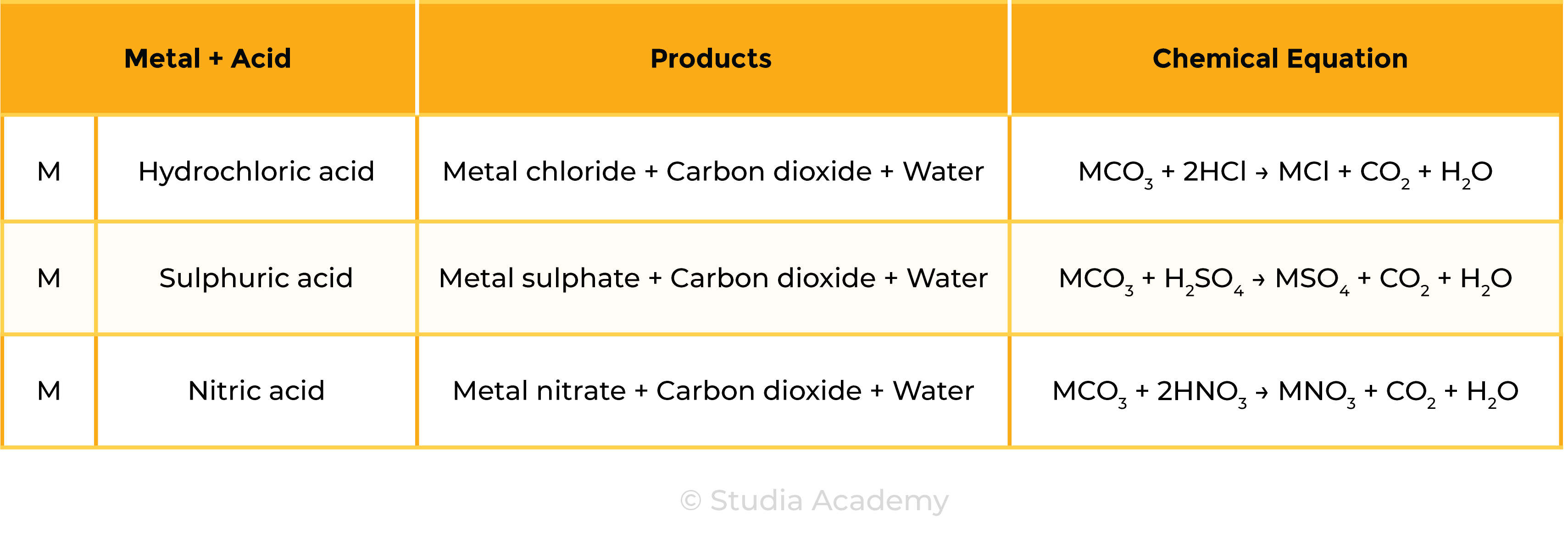
Examples
- 2HCl + Na2CO3 → 2NaCl + H2O + CO2
- H2SO4 + CaCO3 → CaSO4 + H2O + CO2
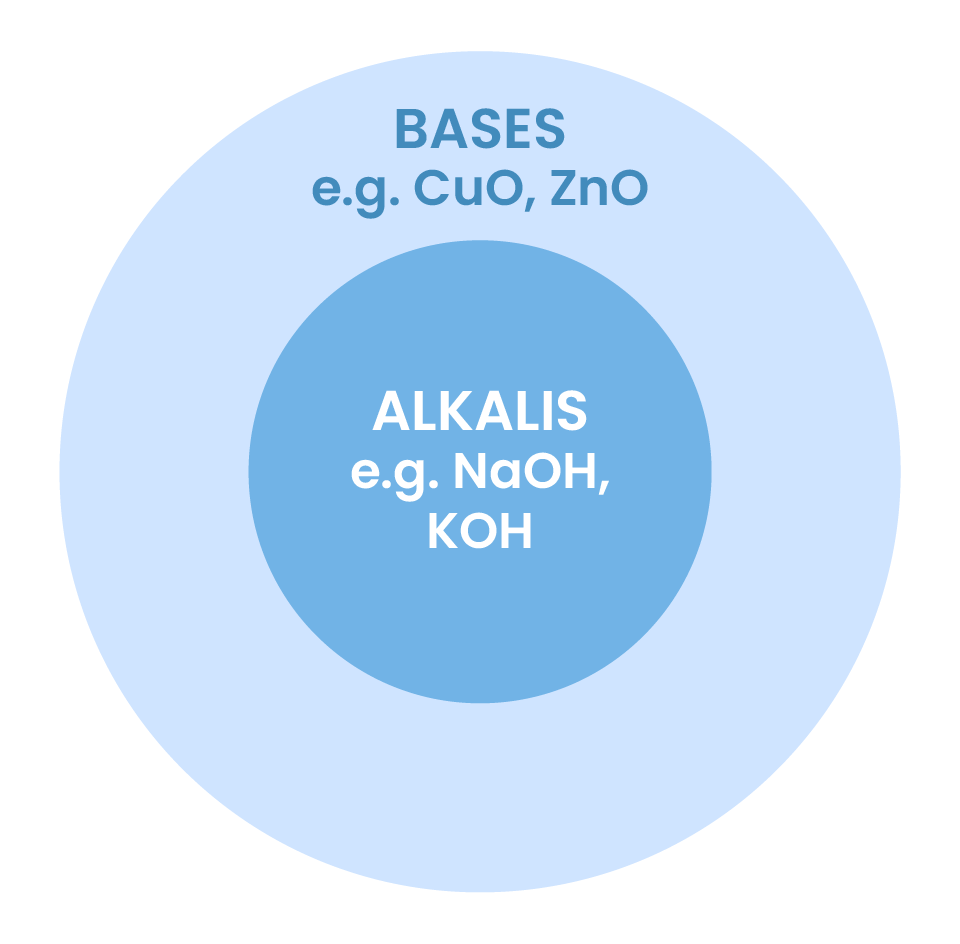
2.7.5 Know that metal oxides, metal hydroxides and ammonia can act as bases, and that alkalis are bases that are soluble in water
Common bases
- Metal oxides (e.g. CuO)
- Metal hydroxides (e.g. NaOH, KOH)
- Ammonia (NH3)
Alkalis
- Bases soluble in water
- OH– donors
- E.g. NaOH, KOH
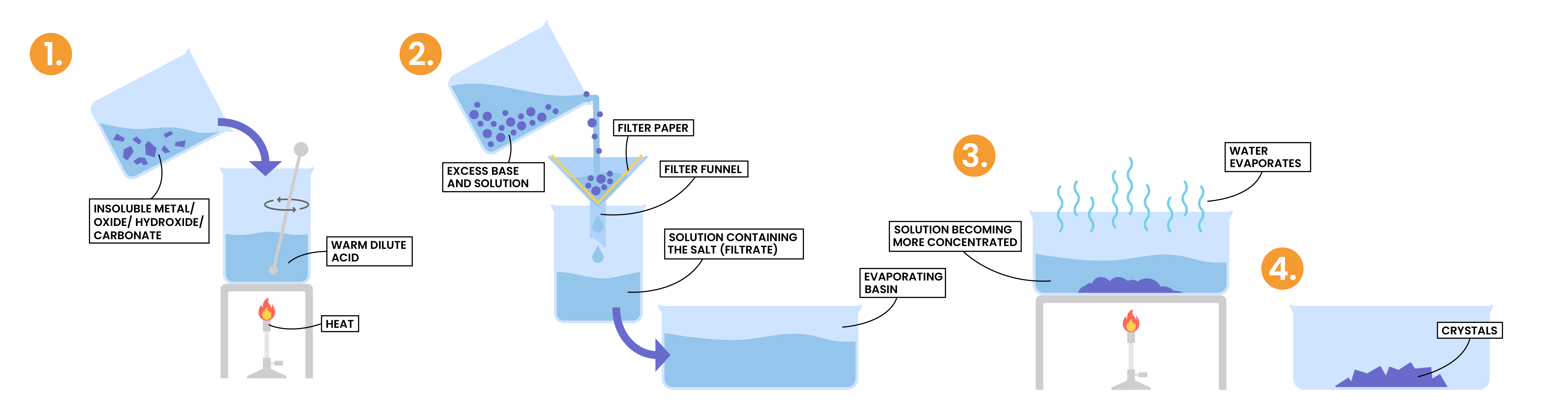
2.7.6 Describe an experiment to prepare a pure, dry sample of a soluble salt, starting from an insoluble reactant
PREPARATION OF SALT
- Soluble salt
- Method 1: from insoluble reactant
- Method 2: from acid and alkali
- Insoluble salt
- Method: from two soluble reactants
- Insoluble reactant is added in excess to the acid
- This is to ensure that all of the acid has reacted
- If this step is not completed, any unreacted acid would become dangerously concentrated during evaporation and crystallisation
- The excess insoluble reactant is then removed by filtration to ensure that only the salt and water remain
- The solution is then heated so water is evaporated and salts are crystallised
- The crystals are then allowed to dry
Key points
- Insoluble base used can be an oxide, hydroxide or carbonate
- When a carbonate is used, carbon dioxide will be produced
- Metal could also be used to react with acid
- However, the metal has to be more reactive than hydrogen while not too reactive so as to prevent dangerously vigorous reaction
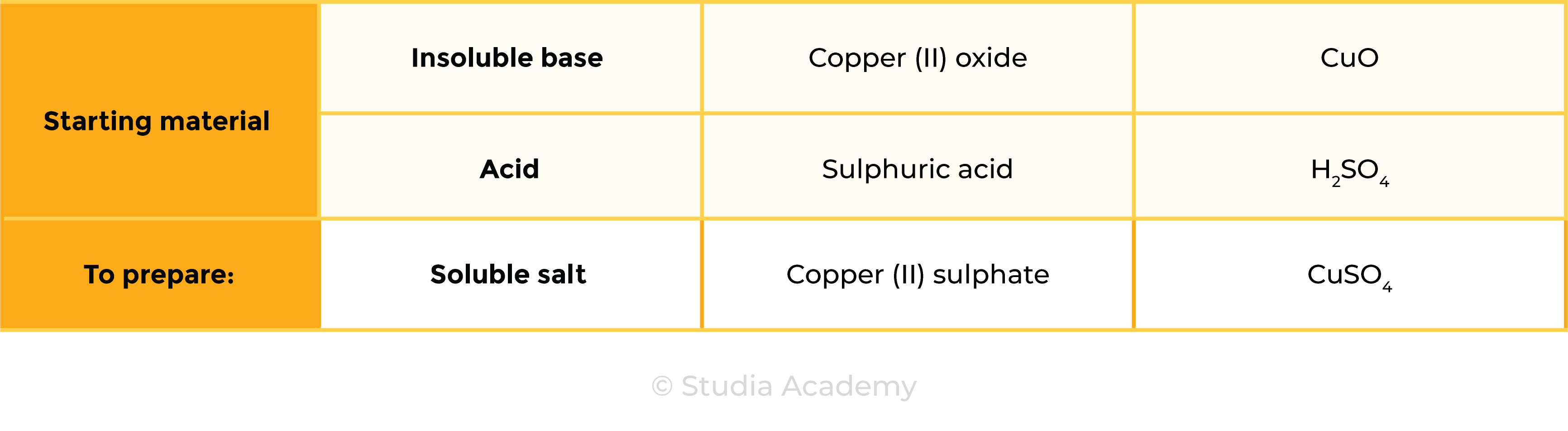
Example: preparation of copper (II) sulphate
CuO (s) + H2SO4 (aq) ⟶ CuSO4 (s) + H2O (l)
- Acid: sulphuric acid (H2SO4)
- Insoluble base: copper (II) oxide (CuO)
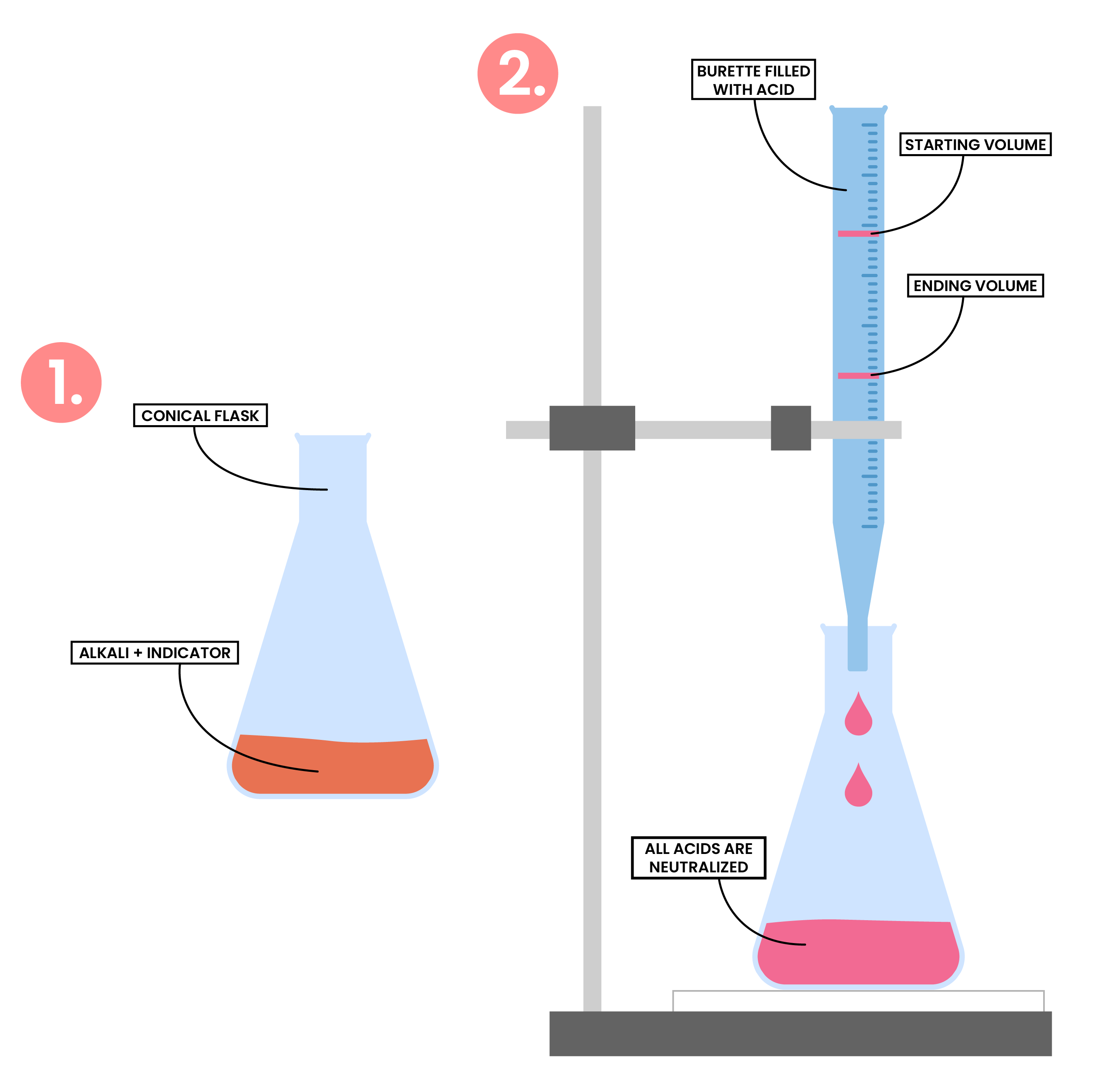
2.7.7C Describe an experiment to prepare a pure, dry sample of a soluble salt, starting from an acid and alkali
PREPARATION OF SOLUBLE SALT METHOD 2
- Measure a known volume of alkali using a measuring cylinder and add it to a conical flask
- Add a few drops of indicator to the conical flask
- Add acid to the burette and record the initial volume
- Perform titration, slowly add the acid from the burette to the conical flask
- Stop immediately when the indicator changes colour
- Record the final volume of the acid
- Calculate the volume of acid reacted with the alkali (initial volume – final volume)
- Add same volume of acid into the same volume of alkali (but without the indicator)
- Heat the solution in an evaporating basin to allow evaporation of water and crystallisation of salt
- The crystals are then allowed to dry
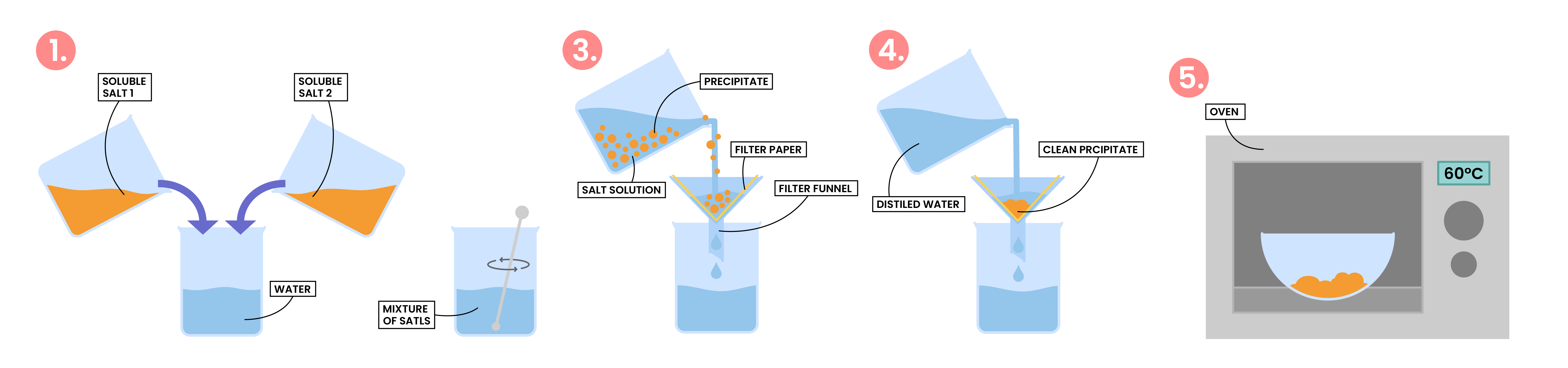
2.7.8C Describe an experiment to prepare a pure, dry sample of an insoluble salt, starting from two soluble reactants
PREPARATION OF INSOLUBLE SALT
- Insoluble salts can be prepared using a precipitation reaction
- The solid salt being formed must be insoluble in water
Soluble Salt 1 + Soluble Salt 2 ⟶ Insoluble Salt + Soluble Salt 3
AB + CD ⟶ AD + CB
- Measure a fixed volume of soluble salt solution 1
- Add soluble salt solution 2 until it is slightly in excess
- This ensures the maximum amount of precipitate will be obtained
- The reaction mixture is then filtered to obtain the precipitate
- Wash the precipitate with water to remove soluble impurities
- The crystals are then warmed in an oven to dry
Example: preparation of silver and lead (II) salts
- Starting material is usually the nitrate of silver or lead(II)
- All nitrates are soluble
2.7.9 Practical: prepare a sample of pure, dry hydrated copper(II) sulfate crystals starting from copper(II) oxide
copper (II) oxide + sulphuric acid → copper (II) sulphate + water
CuO (s) + H2SO4 (aq) ⟶ CuSO4 (s) + H2O (l)
METHODS
- CuO is added in excess to H2SO4
- This is to ensure that all of the acid has reacted
- If this step is not completed, any unreacted acid would become dangerously concentrated during evaporation and crystallisation
- The excess insoluble reactant CuO is then removed by filtration to ensure that only CuSO4 and water remain
- The solution is then heated so water is evaporated and CuSO4 are crystallised
- The CuSO4 crystals are then warmed in an oven to dry
RESULTS
- Hydrated copper(II) sulphate crystals should be bright blue and regularly shaped
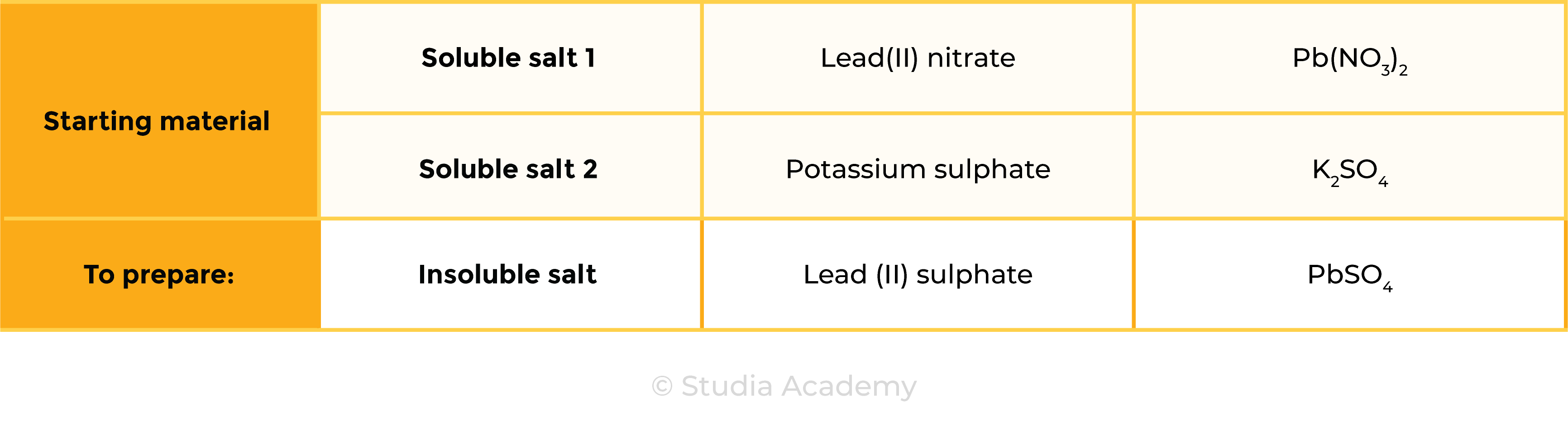
2.7.10C Practical: prepare a sample of pure, dry lead(II) sulfate
lead(II) nitrate + potassium sulphate → lead(II) sulphate + potassium nitrate
Pb(NO3)2 (aq) + K2SO4 (aq) → PbSO4 (s) + 2KNO3 (aq)
METHODS
- Measure a fixed volume of Pb(NO3)2 solution
- Add K2SO4 solution until it is slightly in excess
- This ensures the maximum amount of precipitate will be obtained
- The reaction mixture is then filtered to obtain the precipitate PbSO4
- Wash the PbSO4 precipitate with water to remove soluble impurities
- The PbSO4 crystals are then warmed in an oven to dry

