REVISION NOTES
IGCSE Edexcel Chemistry
2.2 Group 7 (Halogens) – Chlorine, Bromine and Iodine
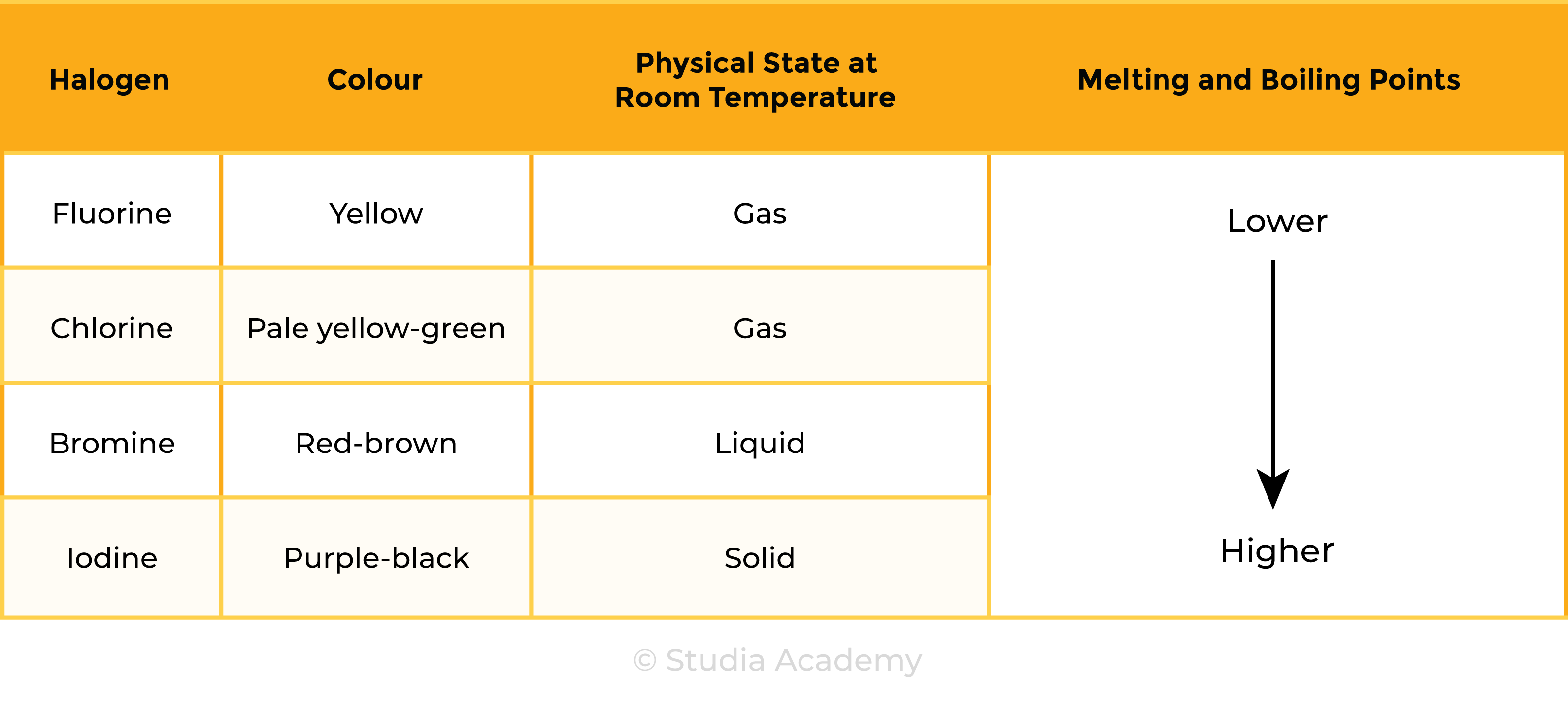
2.2.1 Know the colours, physical states (at room temperature) and trends in physical properties of these elements
GROUP 7 ELEMENTS
- They are known as halogens
- Poisonous non-metals
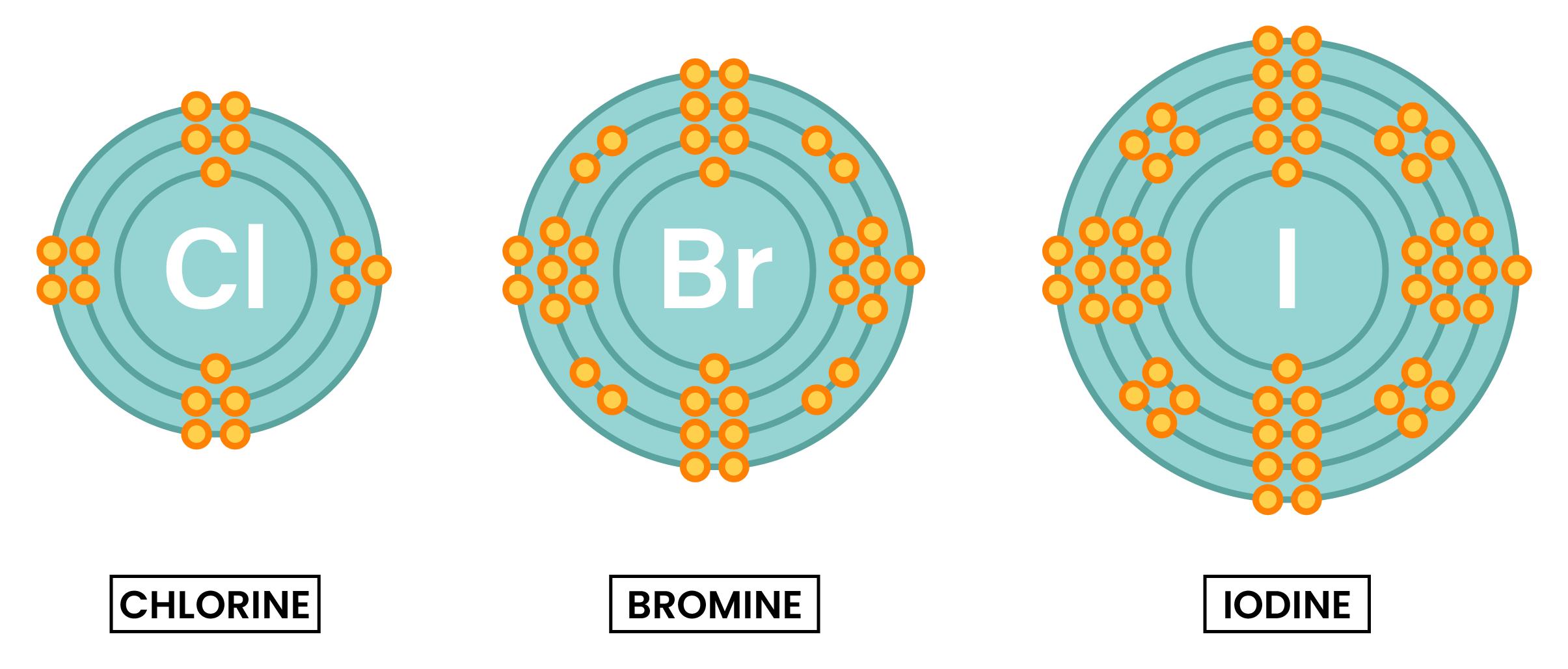
- Similar chemical properties because they have the same number of electrons in the outer shell
- Halogens are diatomic, meaning they form molecules with two halogen atoms sharing electrons
Trends in physical properties
TREND 1:
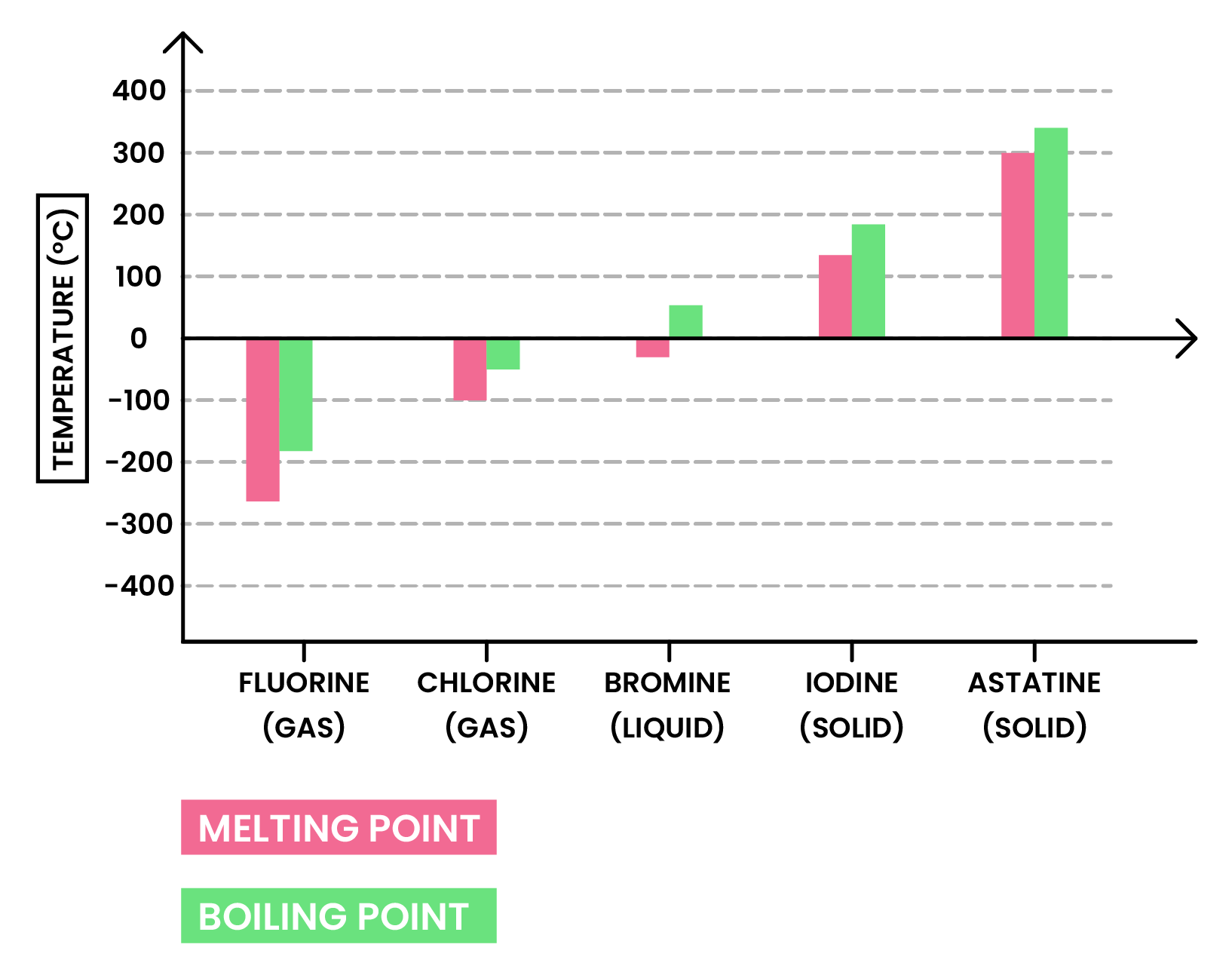
Melting and boiling points increase down the group
- Size of halogen molecules increases down the group
- Increasing intermolecular forces
- More energy is required to overcome the forces
TREND 2
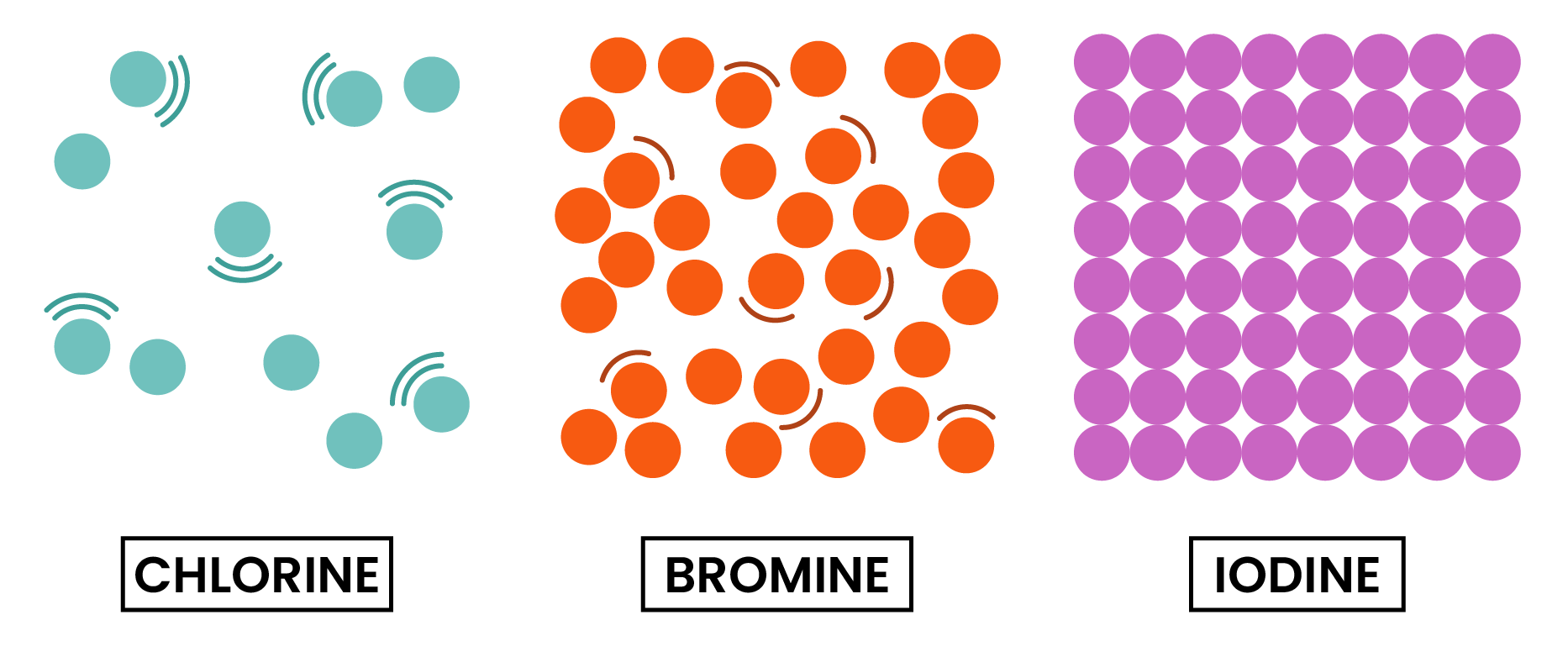
At room temperature, the physical state of halogens changes down the group
- This is also due to the increasing intermolecular forces
TREND 3
Colours of halogens changes down the group
- Becomes darker
2.2.2 Use knowledge of trends in Group 7 to predict the properties of other halogens
RECALL: (DOWN THE GROUP)
- Melting and boiling points increase
- Colour becomes darker
- Physical state at room temperature starts from gas, to liquid, to solid
Halogens can also react with metals and non–metals to form compounds
- Halogen + Metal
- Halogen + Non–metal
- This can be used to predict the properties of other halogens as they share similar chemical properties
REACTION 1 HALOGEN + METAL
Halogen + Metal → Metal halide (salt, ionic compound)
- Metals lose electrons from the valence shell
- The electron(s) is
gained by the halogen, forming halide ions with 1– charge - E.g. sodium + chlorine → sodium chloride
2Na + Cl2 → 2NaCl
- E.g. calcium + bromine → calcium bromide
Ca + Br2 → CaBr2
REACTION 2 HALOGEN + NON-MENTAL
Halogens + Non-metal → simple covalent compounds
- Halogens are also non-metals
- Non–metals tend to gain electron(s) to become more stable
- When non–metals bond together, they share electrons and form covalent compounds (Topic 7: Covalent Bonding)
Halogens + Hydrogen → Hydrogen halide
E.g. chlorine + hydrogen → hydrogen chloride
Cl2 + H2 → 2HCl
2.2.3 Understand how displacement reactions involving halogens and halides provide evidence for the trend in reactivity in Group 7
HALOGEN DISPLACEMENT REACTION
- A more reactive halogen can displace a
less reactive halide in an aqueous solution- E.g. Cl2 can displace Br– ions in a solution to become Cl–
- Whether or not the reaction can happen tells us which halogen is more reactive
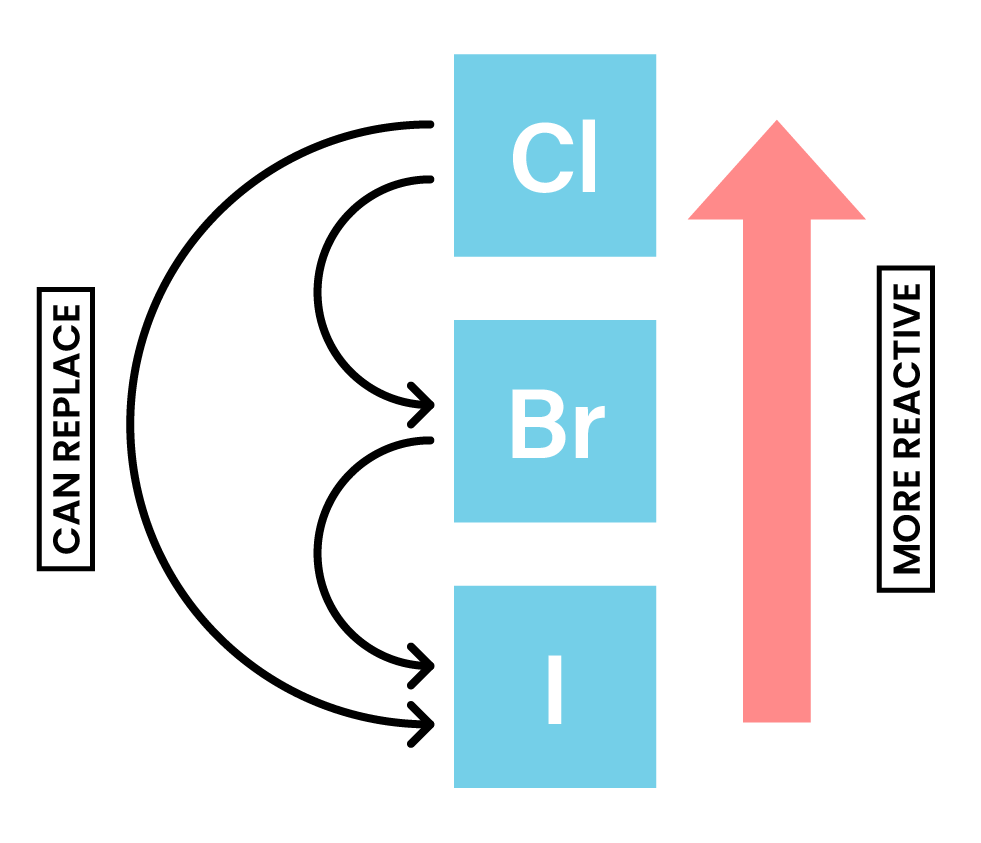
REACTIVITY OF HALOGENS
- The reactivity decreases down the group
- Only reactions with chlorine, bromine and iodine will be studied
- Reactivity Cl > Br > I, which means:
- Chlorine can displace bromide and iodide
- Bromide can displace iodide
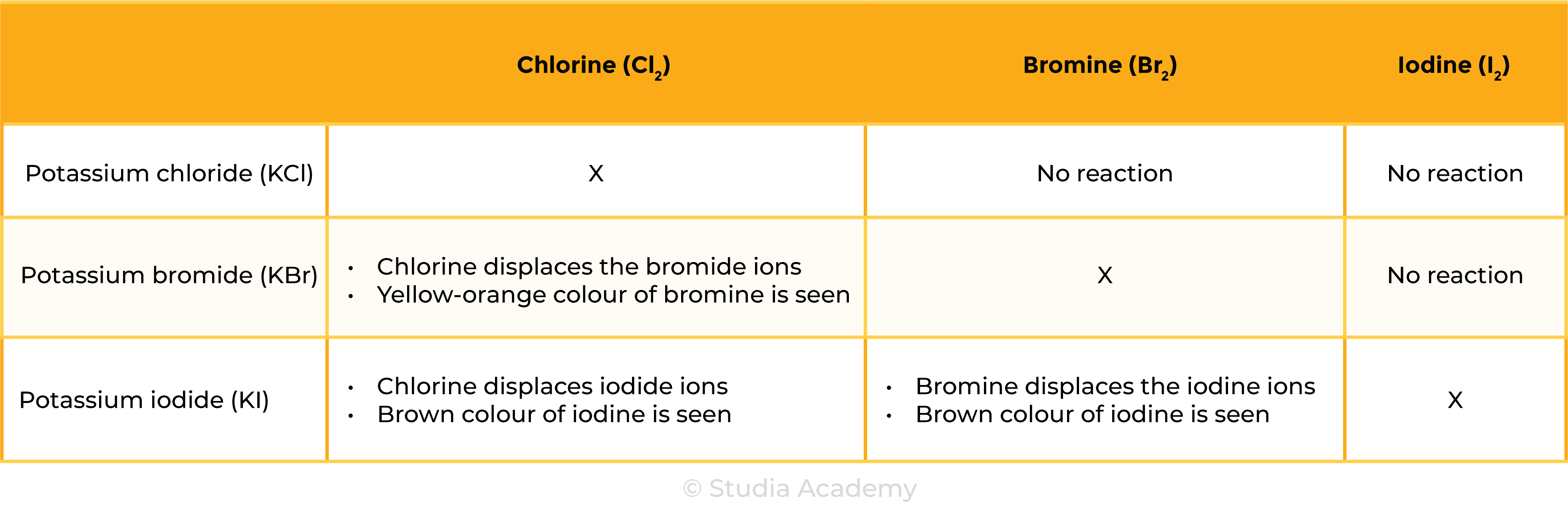
Chlorine with bromides & iodides
- Chlorine will displace bromide or iodide ions in a solution:
Cl2 + 2KBr → 2KCl + Br2
Chlorine + Potassium bromide → potassium chloride + Bromine
Cl2 + 2KI → 2KCl + I2
Chlorine + Potassium iodide→ potassium chloride + Iodine
Bromine with iodides
- Bromine will displace iodide ions in a solution:
Br2 + 2KI → 2KBr + I2
Bromine + Potassium iodide → potassium bromide + Iodine
2.2.4C Explain the trend in reactivity in Group 7 in terms of electronic configurations
RECALL
- The reactivity of group 7 decreases down the group
WHEN HALOGENS REACT
- Halogens have 7 electrons in the outer shell
- When halogens react, they only need to gain one electron
- As a result, it forms an anion with 1– charge
EXPLANATION OF TREND IN REACTIVITY
- Reactivity of halogen depends on how easily the electron is gained