REVISION NOTES
IGCSE Edexcel Biology
2.3 Biological Molecules
2.3.1 Identify the chemical elements present in carbohydrates, proteins and lipids (fats and oils)
Chemical elements in biological molecules:
- Carbohydrates and Lipids contain:
- Carbon
- Oxygen
- Hydrogen
- Proteins contain:
- Carbon
- Oxygen
- Hydrogen
- Proteins contain:
- Nitrogen
- Small amounts of other elements
2.3.2 Describe the structure of carbohydrates, proteins and lipids as large molecules made up from smaller basic units: starch and glycogen from simple sugars, protein from amino acids, and lipid from fatty acids and glycerol
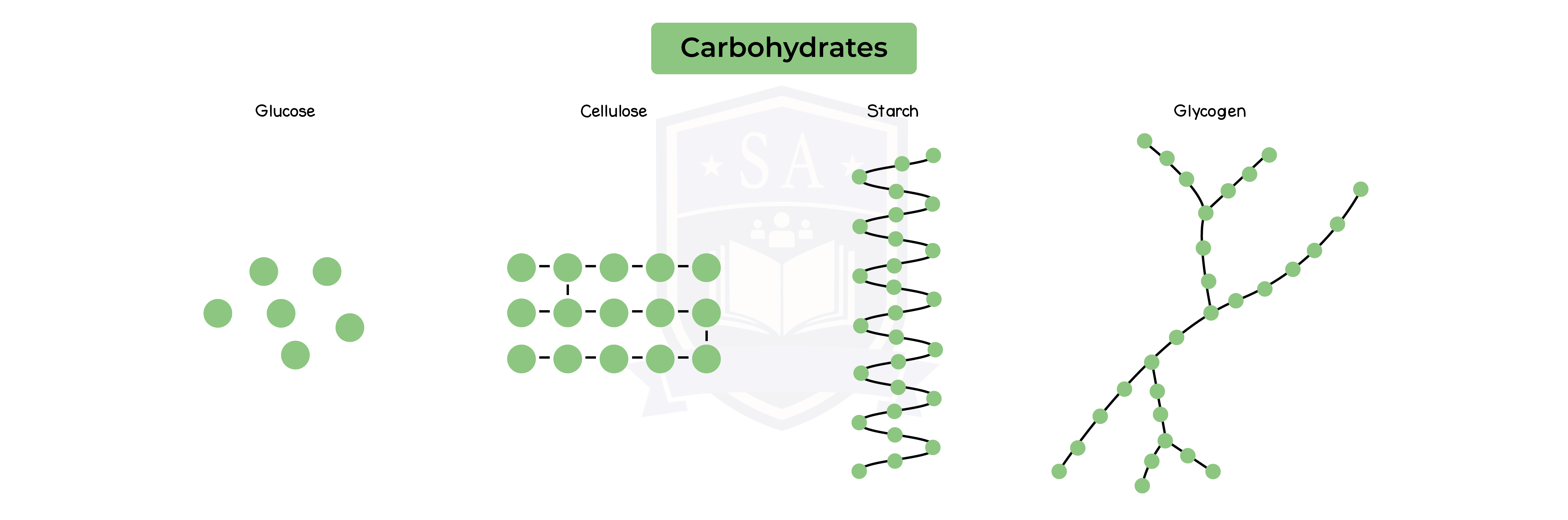
Structure of Carbohydrates:
- Monosaccharides are simple sugars:
- Glucose (C6H12O6)
- Fructose
- Disaccharide are two monosaccharides joined together
- Maltose is made up of two glucose molecules
- Sucrose is made up of one glucose and one fructose molecule
- Lactose
- Polysaccharides are more than two monosaccharides joined together
- Starch
- Glycogen
- Cellulose
- All 3 polysaccharides are made up of multiple glucose molecules joined together
- Polysaccharides are insoluble and used for storage
Structure of Fats:
- Lipids are made from three fatty acid chains bound to a glycerol molecule
- Vary in size and structure based on:
- Length of the fatty acid chain
- Saturated or unsaturated
- Fats are solids at room temperature
Oils are liquids at room temperature
Structure of Proteins:
- Proteins are made up of multiple amino acids bound to each other
- 20 amino acids that can be used for protein production
- Order of amino acids determine the protein being made
- Amino acid sequence contributes to 3-D shape and function
- Examples of proteins:
- Enzymes
- Haemoglobin
- Ligaments
- Keratin
2.3.3 Practical: investigate food samples for the presence of glucose, starch, protein and fat
All food samples should be prepared before conducting any food tests
- Chop the food into smaller pieces
- Transfer food to a test tube and add distilled water
- Mix the food and water by stirring
- Run the mixture through a filter paper and collect the solution
- Use the solution for food tests
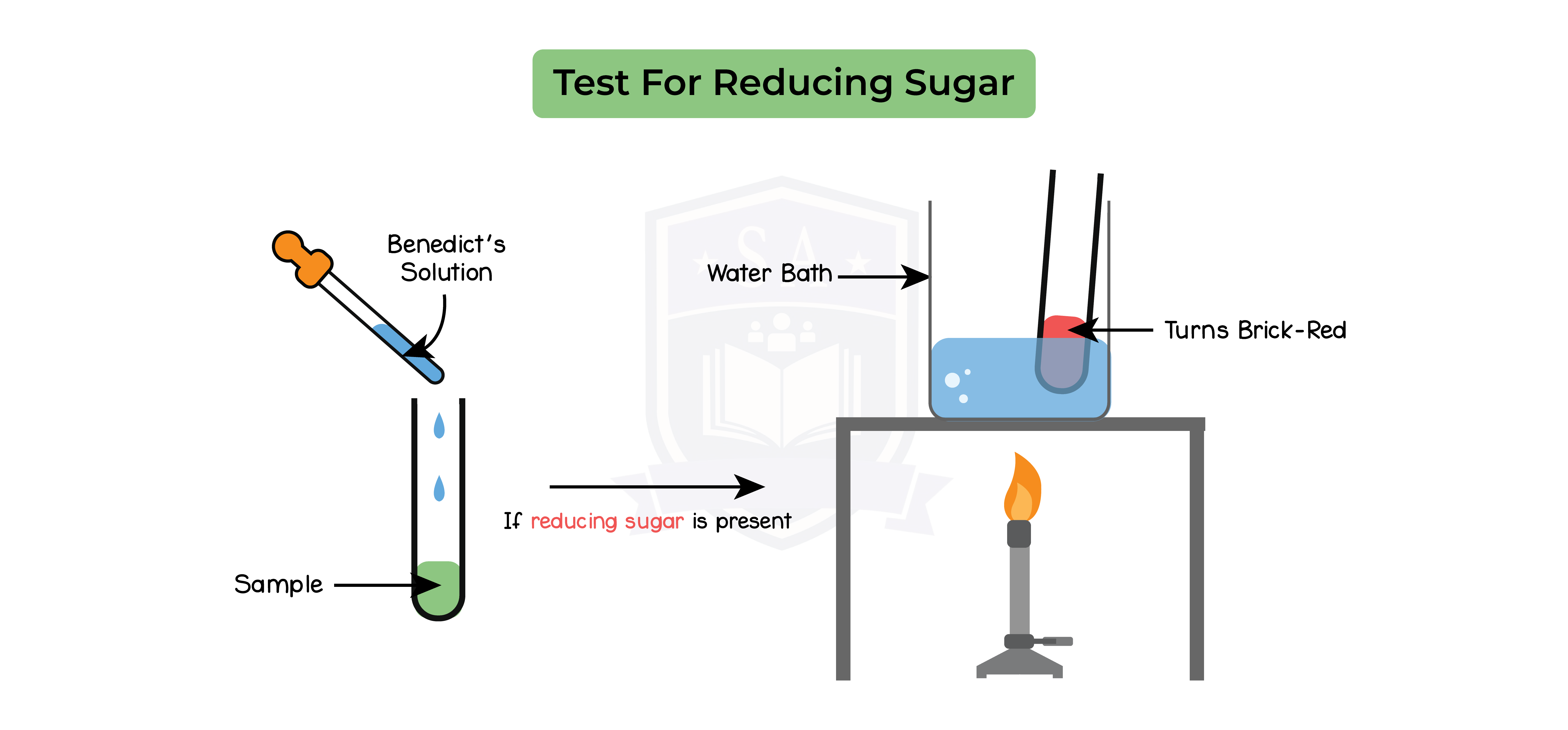
Glucose test:
- Add benedict’s solution to food sample in a test tube
- Heat up the test tube in a water bath for 5-10 mins till the temperature is above 50oC
- Remove the test tube and observe the colour
- Repeat step 1-2 two more times
Results:
- Solution turns red or orange in the presence of glucose
- Solution remains blue in the absence of glucose
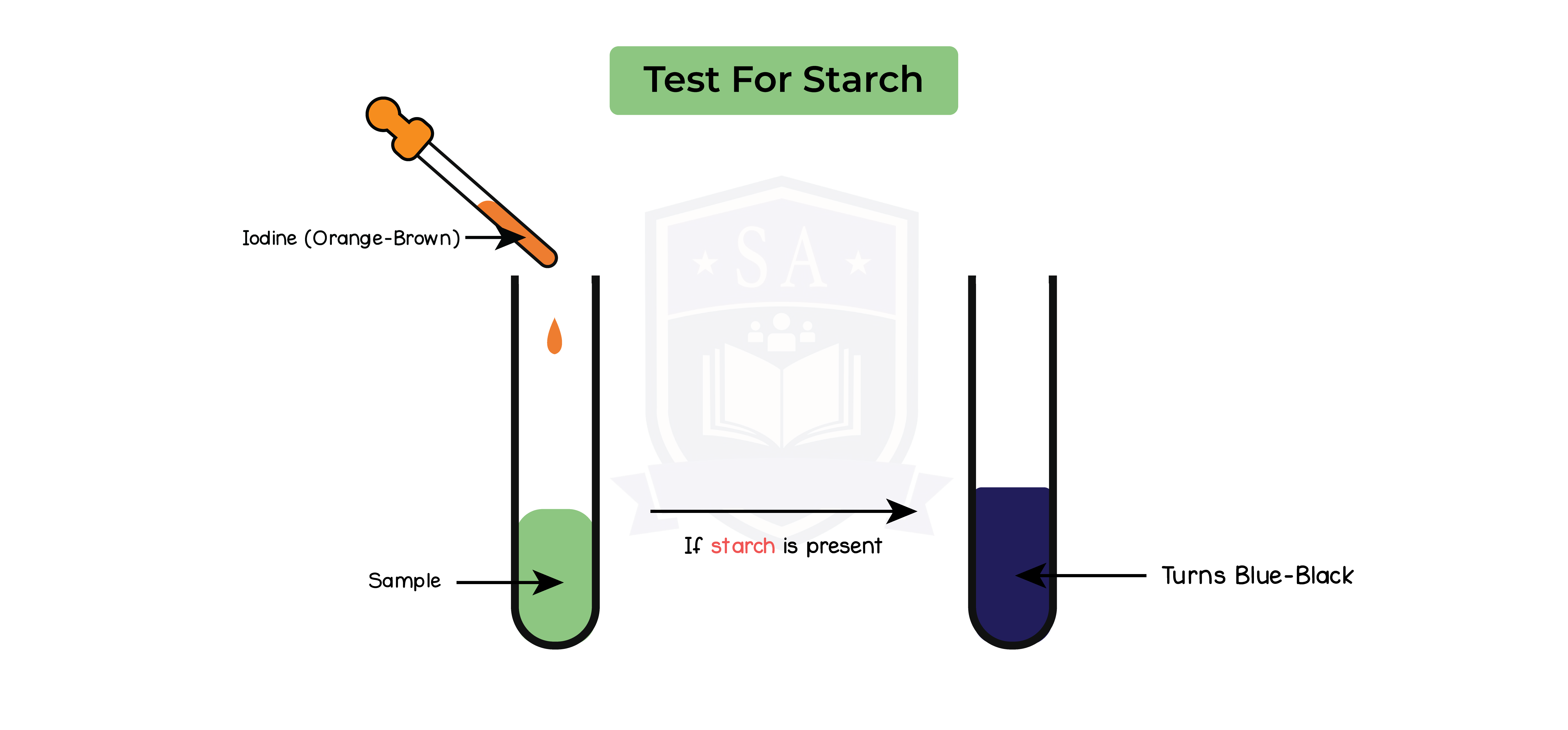
Starch test:
- Add a few drops of iodine solution to the food sample
- Record colour of solution after 2 mins
- Repeat step 1-2 two more times
Results:
- Solution turns blue or black in the presence of starch
- Solution remains orange or brown in the absence of starch
Protein test:
- Add a few drops of Biuret solution to the food sample
- Record colour of solution after 2 mins
- Repeat step 1-2 two more times
Results:
- Solution turns violet or purple in the presence of protein
- Solution remains blue in the absence of protein
Lipid test:
- Add 3cm3 of ethanol to the food sample and properly mix it
- Let the sample dissolve into the ethanol
- Strain the ethanol solution into a test tube and add 3cm3 of distilled water
- Repeat steps 1-3 two more times
Results:
- Solution becomes cloudy in the presence of lipids
- Solution remains clear in the absence of lipids

2.3.4 Understand the role of enzymes as biological catalysts in metabolic reactions
Enzymes:
- Enzymes are biological catalysts for cellular reactions
- Are proteins
- Speed up the rate of reaction
- Are not changed or used up
- Involved in metabolic reactions
- Required to maintain speed of metabolic reactions
- E.g. ensure digestion occurs in 4-6 hours
- Products of enzymatic reactions can be reactants in other reactions
Enzymatic reactions:
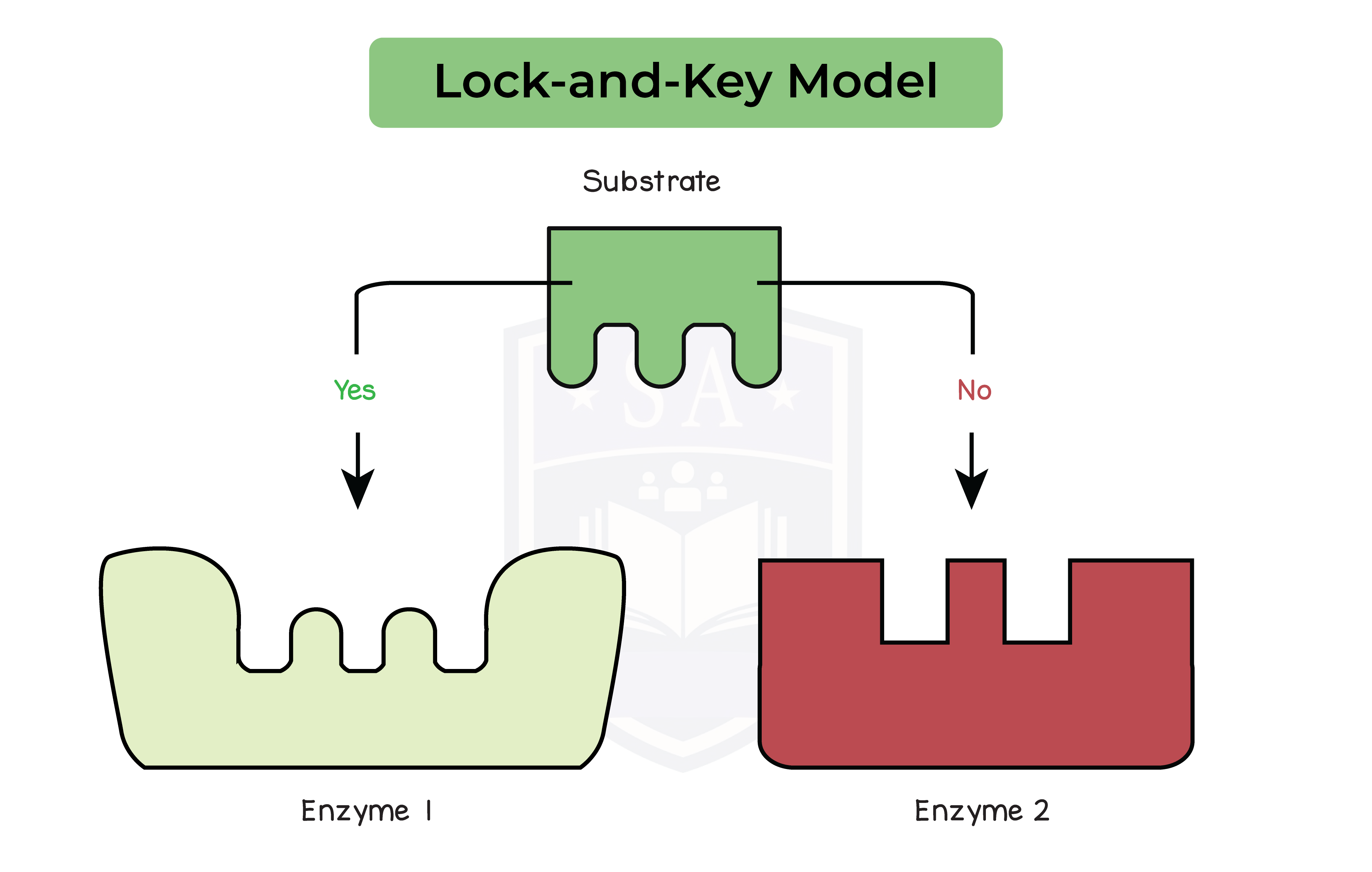
- Enzymes are specific to substrate
- Based on the shape of the active site
- Active site is complementary to the shape of the substrate
- Shape of the enzyme and substrate is based on amino acid sequence
Order of enzymatic reactions:
- Enzymes and substrates randomly move around in a solution
- They randomly collide to form enzyme-substrate complex
- Enzyme-substrate complex formation leads to reaction
- Product(s) formed are released from the active site
- Enzyme remains unchanged and can catalyse other reactions
2.3.5 Understand how temperature changes can affect enzyme function, including changes to the shape of active site
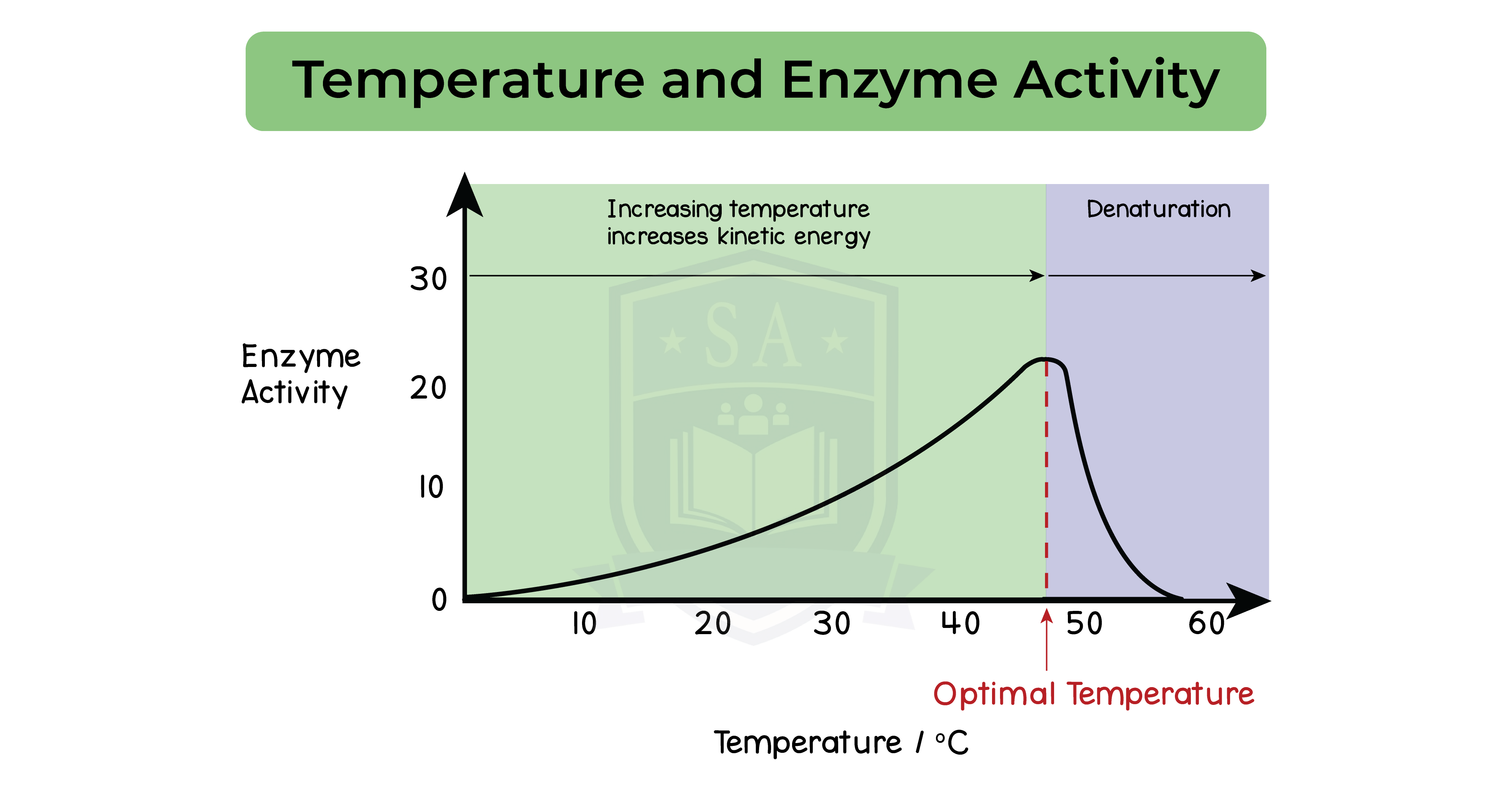
Effect of temperature on enzymatic reactions:
- Rate of reaction is fastest at optimum temperature
- Optimum temperature is 37oC
- Enzymes are amino acid polymers
- Shape of the active site determines the reaction it can catalyse
- Temperature increases towards optimum, rate of reaction increases
- Temperature increases beyond optimum, rate of reaction decreases
Enzymes at temperatures above optimum:
- Bonds between the amino acids break
- Enzyme is denatured
- Shape of active site changes
- Substrate can no longer fit
- Metabolic reaction no longer proceeds
- Enzyme activity stops
Enzymes at temperatures below optimum:
- Enzymes do not denature
- They have less kinetic energy at temperatures below optimum
- Less kinetic energy leads to lower chances of colliding with substrate
- Slower rate of reaction
- Increase in kinetic energy as temperature increases towards optimum
- Enzymes move faster
Increase in collision with substrates which increases rate of reaction
Irreversible effect

2.3.6 Practical: investigate how enzyme activity can be affected by changes in temperature
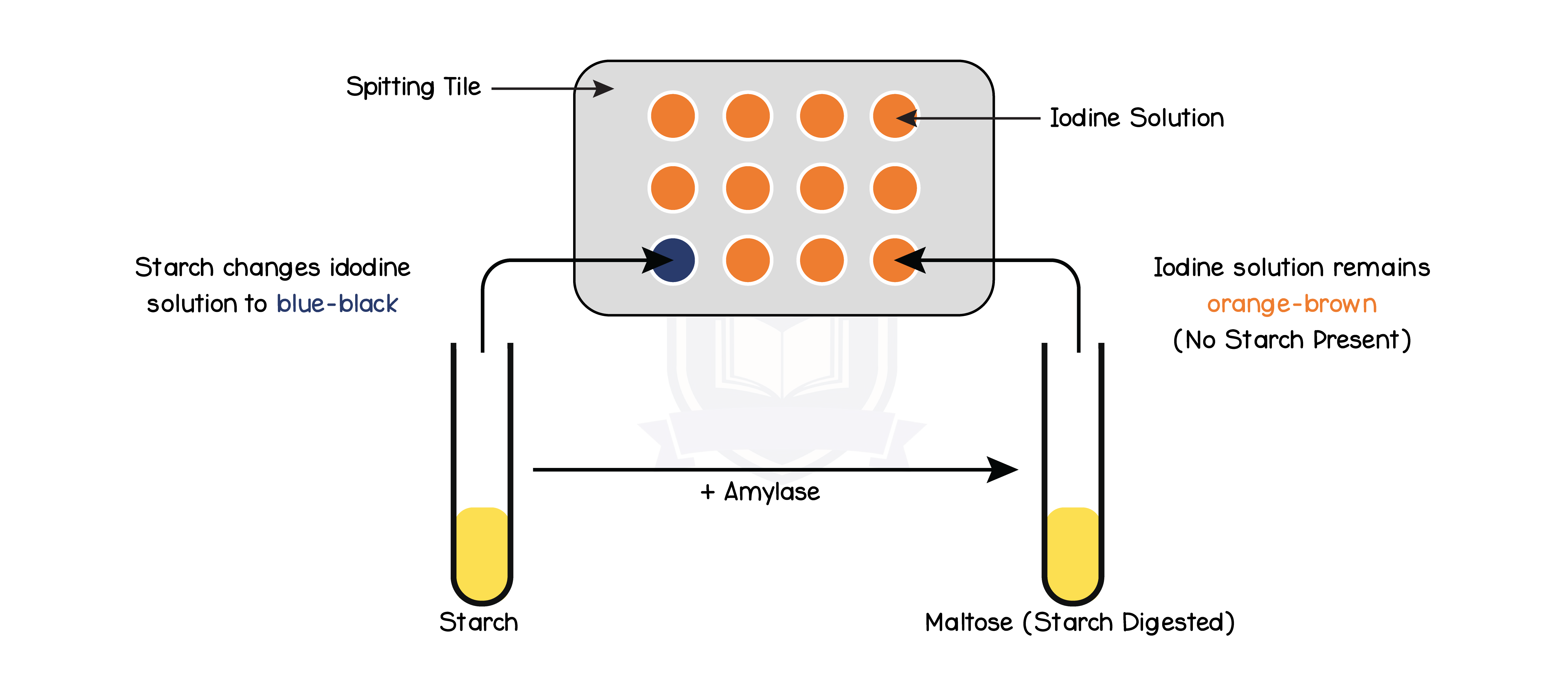
1. Starch is digested into maltose by an enzyme called amylase
Method:
- Add 5ml of starch solution to a test tube that is then heated to a particular temperature in a water bath
- Using a syringe, add 2ml of amylase to the starch solution from step 1
- Add 1 drop of iodine to each well of the spotting tile
- Every minute, transfer one drop of starch-amylase solution to a new well of the spotting tile
- Keep transferring a drop of the starch-amylase solution until the iodine stops turning blue/black (when the amylase has broken down all the starch)
- Record the time taken for all of the starch to be broken down by the amylase
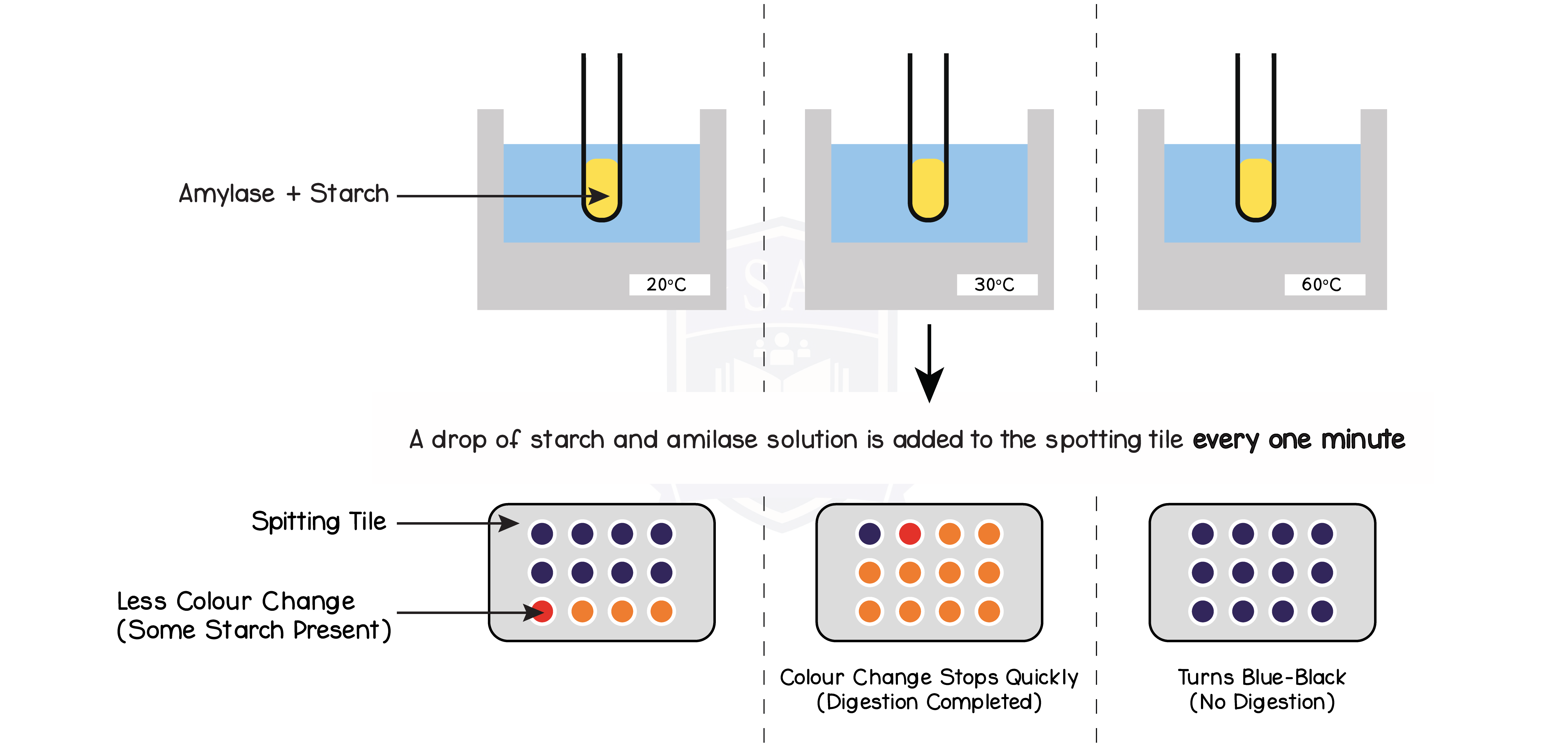
- Repeat this experiment for a range of different temperatures
Results:
- Amylase breaks down starch, thus the faster the enzyme is working, the quicker the iodine will stop turning blue/black
- At the optimum temperature, the iodine stops turning blue/black the fastest
- Enzyme work at their fastest rate at optimum temperatures so starch is digested faster
- At temperatures below optimum, the iodine takes a longer time turning blue/black
- Enzymes has less kinetic energy and thus have a decreased rate of reaction
- At temperatures above optimum, the iodine stays blue/black throughout the experiment
- Enzyme denatures and can no longer breakdown starch
Control Variables:
- Temperature
- Water baths at each temperature
- Re-confirm using a thermometer
- Change in iodine solution colour when compared to a control
- Colorimeter to accurately measure colour change
2.3.7 Understand how enzyme function can be affected by changes in pH altering the active
site
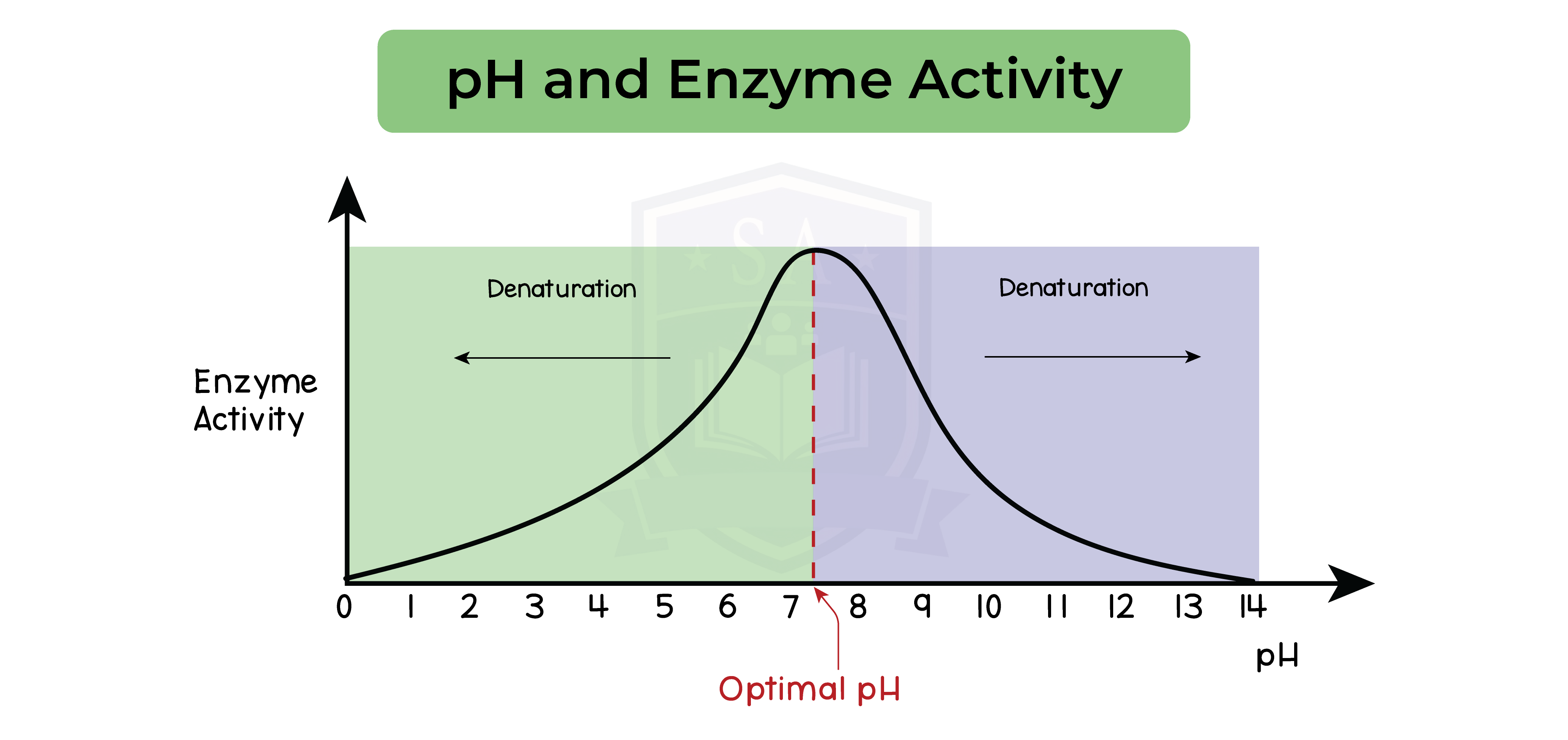
- Optimum pH for most enzymes is 7
- Exceptions are:
- Stomach enzymes have an optimum pH of 2
- Enzymes in the duodenum have an optimum pH of 8 or 9
- pH is above or below optimum
- Break the bonds within the amino acid chain
- Changes the shape of the active site
- pH is significantly away from the optimum pH
- Enzyme denaturation
- Stops all enzymatic activity
2.3.8B Practical: investigate how enzyme activity can be affected by changes in pH
Method:
- Add 1 drop of iodine to each well of a spotting tile or tray
- Add 4cm3 of amylase solution into a test tube
- Add 4cm3 of starch to the test tube
- Add 2cm3 of buffer solution of the testing pH to the test tube
- Mix the solution using a glass rod
- Start a stopwatch and transfer a droplet of the solution to a well of spotting tile after every 20s
- Continue transferring a drop of the solution into a spotting tile for 5 mins
- Record the time taken for all of the starch to be broken down by the amylase
- The colour of the solution remains orange/brown
- Repeat this experiment for a range of different pH levels
Results:
- At the optimum pH, the iodine stops turning blue/black the fastest
- Enzyme works at its fastest rate at optimum pH and so starch is digested fastest
- At pH above or below optimum, the iodine takes a longer time turning blue/black
- Enzyme starts denaturing as a result of pH above or below optimum and thus is unable to bind to starch and break it down